
Material properties (thermodynamics)
Encyclopedia
The thermodynamic properties of materials are intensive thermodynamic parameters which are specific to a given material. Each is directly related to a second order differential of a thermodynamic potential. Examples for a simple 1-component system are:
- Compressibility (or its inverse, the bulk modulusBulk modulusThe bulk modulus of a substance measures the substance's resistance to uniform compression. It is defined as the pressure increase needed to decrease the volume by a factor of 1/e...
) - Isothermal compressibility
-
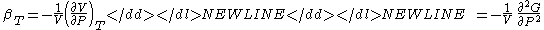
- Adiabatic compressibility
-
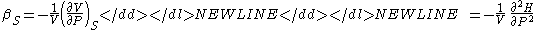
- Specific heat (Note - the extensive analog is the heat capacityHeat capacityHeat capacity , or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a substance's temperature by a given amount...
) - Specific heat at constant pressure
-
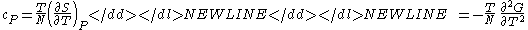
- Specific heat at constant volume
-
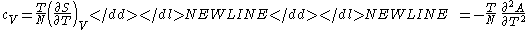
- Coefficient of thermal expansionThermal expansionThermal expansion is the tendency of matter to change in volume in response to a change in temperature.When a substance is heated, its particles begin moving more and thus usually maintain a greater average separation. Materials which contract with increasing temperature are rare; this effect is...
-
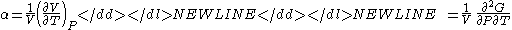
where P is pressurePressurePressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
, V is volume, T is temperatureTemperatureTemperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
, S is entropyEntropyEntropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
, and N is the number of particlesParticle numberThe particle number of a thermodynamic system, conventionally indicated with the letter N, is the number of constituent particles in that system. The particle number is a fundamental parameter in thermodynamics which is conjugate to the chemical potential. Unlike most physical quantities, particle...
.
For a single component system, only three second derivatives are needed in order to derive all others, and so only three material properties are needed to derive all others. For a single component system, the "standard" three parameters are the isothermal compressibility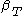 , the specific heat at constant pressure
, the specific heat at constant pressure 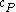 , and the coefficient of thermal expansion
, and the coefficient of thermal expansion  .
.
For example, the following equations are true:
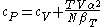
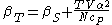
The three "standard" properties are in fact the three possible second derivatives of the Gibbs free energyGibbs free energyIn thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
with respect to temperature and pressure.
Sources
The Dortmund Data BankDortmund Data BankThe Dortmund Data Bank is a factual data bank for thermodynamic and thermophysical data. Its main usage is the data supply for process simulation where experimental data are the basis for the design, analysis, synthesis, and optimization of chemical processes...
is a factual data bank for thermodynamic and thermophysical data.
See thermodynamic databases for pure substancesThermodynamic databases for pure substancesThermodynamic databases contain information about thermodynamic properties for substances, the most important being enthalpy, entropy, and Gibbs free energy. Numerical values of these thermodynamic properties are collected as tables or are calculated from thermodynamic datafiles...
.
- Coefficient of thermal expansion
- Specific heat (Note - the extensive analog is the heat capacity

