
Metabolic acidosis
Encyclopedia
In medicine
, metabolic acidosis is a condition that occurs when the body produces too much acid or when the kidney
s are not removing enough acid from the body. If unchecked, metabolic acidosis leads to acidemia, i.e., blood pH
is low (less than 7.35) due to increased production of hydrogen
by the body or the inability of the body to form bicarbonate
(HCO3-) in the kidney. Its causes are diverse, and its consequences can be serious, including coma
and death
. Together with respiratory acidosis
, it is one of the two general causes of acidemia.
In most cases, acidosis occurs first for reasons explained below. Free hydrogen ions then diffuse into the blood, lowering the pH. Arterial blood gas
analysis detects acidemia (pH lower than 7.35). When acidemia is present, acidosis is presumed.
sampling. Symptoms may include chest pain
, palpitations, headache
, altered mental status such as
severe anxiety due to hypoxia
, decreased visual acuity, nausea
, vomiting
, abdominal pain
, altered appetite (either loss of or increased) and weight loss
(longer term), muscle weakness
and bone pain
s. Those in metabolic acidosis may exhibit deep, rapid breathing called Kussmaul respirations which is classically associated with diabetic ketoacidosis
. Rapid deep breaths increase the amount of carbon dioxide exhaled, thus lowering the serum carbon dioxide levels, resulting in some degree of compensation. Over compensation via respiratory alkalosis to form an alkalemia does not occur.
Extreme acidemia leads to neurological and cardiac complications:
Physical examination
occasionally reveals signs of disease, but is otherwise normal. Cranial nerve abnormalities are reported in ethylene glycol
poisoning, and retina
l edema
can be a sign of methanol
(methyl alcohol) intoxication. Longstanding chronic metabolic acidosis leads to osteoporosis
and can cause fracture
s.
sampling is essential for the diagnosis. If the pH is low (under 7.35) and the bicarbonate levels are decreased (<24 mmol/l), metabolic acidemia is present, and metabolic acidosis is presumed. Due to respiratory compensation (hyperventilation), carbon dioxide is decreased and conversely oxygen is increased. An ECG can be useful to anticipate cardiac complications.
Other tests that are relevant in this context are electrolyte
s (including chloride
), glucose
, renal function
and a full blood count. Urinalysis
can reveal acidity (salicylate poisoning) or alkalinity (renal tubular acidosis type I). In addition, it can show ketones in ketoacidosis.
To distinguish between the main types of metabolic acidosis, a clinical tool called the anion gap
is considered very useful. It is calculated by subtracting the chloride and bicarbonate levels from the sodium.
Anion gap = ( [Na+] ) - ( [Cl-]+[HCO3-] )
As sodium is the main extracellular cation, and chloride and bicarbonate are the main anions, the result should reflect the remaining anions. Normally, this concentration is about 8-16 mmol/l (12±4). An elevated anion gap (i.e. > 16 mmol/l) can indicate particular types of metabolic acidosis, particularly certain poisons, lactate acidosis and ketoacidosis.
As the differential diagnosis
is made, certain other tests may be necessary, including toxicological screening and imaging of the kidneys. It is also important to differentiate between acidosis-induced hyperventilation and asthma; otherwise, treatment could lead to inappropriate bronchodilation.http://www.ncbi.nlm.nih.gov/sites/entrez/17906597?dopt=Abstract&holding=f1000,f1000m,isrctn
.
It bears noting that the anion gap can be spuriously normal in sampling errors of the sodium level, e.g. in extreme hypertriglyceridemia
. The anion gap can be increased due to relatively low levels of cations other than sodium and potassium (e.g. calcium or magnesium).
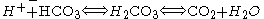
The Henderson-Hasselbalch equation
mathematically describes the relationship between blood pH and the components of the bicarbonate buffering system:
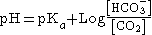

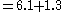
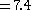
.
If the acidosis is particularly severe and/or there may be intoxication, consultation with the nephrology
team is considered useful, as dialysis
may clear both the intoxication and the acidosis.
Medicine
Medicine is the science and art of healing. It encompasses a variety of health care practices evolved to maintain and restore health by the prevention and treatment of illness....
, metabolic acidosis is a condition that occurs when the body produces too much acid or when the kidney
Kidney
The kidneys, organs with several functions, serve essential regulatory roles in most animals, including vertebrates and some invertebrates. They are essential in the urinary system and also serve homeostatic functions such as the regulation of electrolytes, maintenance of acid–base balance, and...
s are not removing enough acid from the body. If unchecked, metabolic acidosis leads to acidemia, i.e., blood pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
is low (less than 7.35) due to increased production of hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
by the body or the inability of the body to form bicarbonate
Bicarbonate
In inorganic chemistry, bicarbonate is an intermediate form in the deprotonation of carbonic acid...
(HCO3-) in the kidney. Its causes are diverse, and its consequences can be serious, including coma
Coma
In medicine, a coma is a state of unconsciousness, lasting more than 6 hours in which a person cannot be awakened, fails to respond normally to painful stimuli, light or sound, lacks a normal sleep-wake cycle and does not initiate voluntary actions. A person in a state of coma is described as...
and death
Death
Death is the permanent termination of the biological functions that sustain a living organism. Phenomena which commonly bring about death include old age, predation, malnutrition, disease, and accidents or trauma resulting in terminal injury....
. Together with respiratory acidosis
Respiratory acidosis
Respiratory acidosis is a medical condition in which decreased ventilation causes increased blood carbon dioxide concentration and decreased pH ....
, it is one of the two general causes of acidemia.
Terminology
- Acidosis refers to a low pH in tissue.
- Acidemia refers to a low pH in the blood.
In most cases, acidosis occurs first for reasons explained below. Free hydrogen ions then diffuse into the blood, lowering the pH. Arterial blood gas
Arterial blood gas
An arterial blood gas is a blood test that is performed using blood from an artery. It involves puncturing an artery with a thin needle and syringe and drawing a small volume of blood. The most common puncture site is the radial artery at the wrist, but sometimes the femoral artery in the groin or...
analysis detects acidemia (pH lower than 7.35). When acidemia is present, acidosis is presumed.
Signs and symptoms
Symptoms are aspecific, and diagnosis can be difficult unless the patient presents with clear indications for arterial blood gasArterial blood gas
An arterial blood gas is a blood test that is performed using blood from an artery. It involves puncturing an artery with a thin needle and syringe and drawing a small volume of blood. The most common puncture site is the radial artery at the wrist, but sometimes the femoral artery in the groin or...
sampling. Symptoms may include chest pain
Chest pain
Chest pain may be a symptom of a number of serious conditions and is generally considered a medical emergency. Even though it may be determined that the pain is non-cardiac in origin, this is often a diagnosis of exclusion made after ruling out more serious causes of the pain.-Differential...
, palpitations, headache
Headache
A headache or cephalalgia is pain anywhere in the region of the head or neck. It can be a symptom of a number of different conditions of the head and neck. The brain tissue itself is not sensitive to pain because it lacks pain receptors. Rather, the pain is caused by disturbance of the...
, altered mental status such as
severe anxiety due to hypoxia
Hypoxia (medical)
Hypoxia, or hypoxiation, is a pathological condition in which the body as a whole or a region of the body is deprived of adequate oxygen supply. Variations in arterial oxygen concentrations can be part of the normal physiology, for example, during strenuous physical exercise...
, decreased visual acuity, nausea
Nausea
Nausea , is a sensation of unease and discomfort in the upper stomach with an involuntary urge to vomit. It often, but not always, precedes vomiting...
, vomiting
Vomiting
Vomiting is the forceful expulsion of the contents of one's stomach through the mouth and sometimes the nose...
, abdominal pain
Abdominal pain
Abdominal pain can be one of the symptoms associated with transient disorders or serious disease. Making a definitive diagnosis of the cause of abdominal pain can be difficult, because many diseases can result in this symptom. Abdominal pain is a common problem...
, altered appetite (either loss of or increased) and weight loss
Weight loss
Weight loss, in the context of medicine, health or physical fitness, is a reduction of the total body mass, due to a mean loss of fluid, body fat or adipose tissue and/or lean mass, namely bone mineral deposits, muscle, tendon and other connective tissue...
(longer term), muscle weakness
Muscle weakness
Muscle weakness or myasthenia is a lack of muscle strength. The causes are many and can be divided into conditions that have true or perceived muscle weakness...
and bone pain
Bone pain
Bone pain is a debilitating form of pain emanating from the bone tissue. It occurs as a result of a wide range of diseases and/or physical conditions and may severely impair the quality of life for patients who suffer from it...
s. Those in metabolic acidosis may exhibit deep, rapid breathing called Kussmaul respirations which is classically associated with diabetic ketoacidosis
Ketoacidosis
Ketoacidosis is a metabolic state associated with high concentrations of ketone bodies, formed by the breakdown of fatty acids and the deamination of amino acids. The two common ketones produced in humans are acetoacetic acid and β-hydroxybutyrate....
. Rapid deep breaths increase the amount of carbon dioxide exhaled, thus lowering the serum carbon dioxide levels, resulting in some degree of compensation. Over compensation via respiratory alkalosis to form an alkalemia does not occur.
Extreme acidemia leads to neurological and cardiac complications:
- Neurological: lethargy, stupor, comaComaIn medicine, a coma is a state of unconsciousness, lasting more than 6 hours in which a person cannot be awakened, fails to respond normally to painful stimuli, light or sound, lacks a normal sleep-wake cycle and does not initiate voluntary actions. A person in a state of coma is described as...
, seizureSeizureAn epileptic seizure, occasionally referred to as a fit, is defined as a transient symptom of "abnormal excessive or synchronous neuronal activity in the brain". The outward effect can be as dramatic as a wild thrashing movement or as mild as a brief loss of awareness...
s. - Cardiac: arrhythmias (ventricular tachycardiaVentricular tachycardiaVentricular tachycardia is a tachycardia, or fast heart rhythm, that originates in one of the ventricles of the heart...
), decreased response to epinephrineEpinephrineEpinephrine is a hormone and a neurotransmitter. It increases heart rate, constricts blood vessels, dilates air passages and participates in the fight-or-flight response of the sympathetic nervous system. In chemical terms, adrenaline is one of a group of monoamines called the catecholamines...
; both lead to hypotensionHypotensionIn physiology and medicine, hypotension is abnormally low blood pressure, especially in the arteries of the systemic circulation. It is best understood as a physiologic state, rather than a disease. It is often associated with shock, though not necessarily indicative of it. Hypotension is the...
(low blood pressure).
Physical examination
Physical examination
Physical examination or clinical examination is the process by which a doctor investigates the body of a patient for signs of disease. It generally follows the taking of the medical history — an account of the symptoms as experienced by the patient...
occasionally reveals signs of disease, but is otherwise normal. Cranial nerve abnormalities are reported in ethylene glycol
Ethylene glycol
Ethylene glycol is an organic compound widely used as an automotive antifreeze and a precursor to polymers. In its pure form, it is an odorless, colorless, syrupy, sweet-tasting liquid...
poisoning, and retina
Retina
The vertebrate retina is a light-sensitive tissue lining the inner surface of the eye. The optics of the eye create an image of the visual world on the retina, which serves much the same function as the film in a camera. Light striking the retina initiates a cascade of chemical and electrical...
l edema
Edema
Edema or oedema ; both words from the Greek , oídēma "swelling"), formerly known as dropsy or hydropsy, is an abnormal accumulation of fluid beneath the skin or in one or more cavities of the body that produces swelling...
can be a sign of methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
(methyl alcohol) intoxication. Longstanding chronic metabolic acidosis leads to osteoporosis
Osteoporosis
Osteoporosis is a disease of bones that leads to an increased risk of fracture. In osteoporosis the bone mineral density is reduced, bone microarchitecture is deteriorating, and the amount and variety of proteins in bone is altered...
and can cause fracture
Fracture
A fracture is the separation of an object or material into two, or more, pieces under the action of stress.The word fracture is often applied to bones of living creatures , or to crystals or crystalline materials, such as gemstones or metal...
s.
Diagnosis
Arterial blood gasArterial blood gas
An arterial blood gas is a blood test that is performed using blood from an artery. It involves puncturing an artery with a thin needle and syringe and drawing a small volume of blood. The most common puncture site is the radial artery at the wrist, but sometimes the femoral artery in the groin or...
sampling is essential for the diagnosis. If the pH is low (under 7.35) and the bicarbonate levels are decreased (<24 mmol/l), metabolic acidemia is present, and metabolic acidosis is presumed. Due to respiratory compensation (hyperventilation), carbon dioxide is decreased and conversely oxygen is increased. An ECG can be useful to anticipate cardiac complications.
Other tests that are relevant in this context are electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
s (including chloride
Chloride
The chloride ion is formed when the element chlorine, a halogen, picks up one electron to form an anion Cl−. The salts of hydrochloric acid HCl contain chloride ions and can also be called chlorides. The chloride ion, and its salts such as sodium chloride, are very soluble in water...
), glucose
Glucose
Glucose is a simple sugar and an important carbohydrate in biology. Cells use it as the primary source of energy and a metabolic intermediate...
, renal function
Renal function
Renal function, in nephrology, is an indication of the state of the kidney and its role in renal physiology. Glomerular filtration rate describes the flow rate of filtered fluid through the kidney...
and a full blood count. Urinalysis
Urinalysis
A urinalysis , also known as Routine and Microscopy , is an array of tests performed on urine, and one of the most common methods of medical diagnosis...
can reveal acidity (salicylate poisoning) or alkalinity (renal tubular acidosis type I). In addition, it can show ketones in ketoacidosis.
To distinguish between the main types of metabolic acidosis, a clinical tool called the anion gap
Anion gap
The anion gap is the difference in the measured cations and the measured anions in serum, plasma, or urine. The magnitude of this difference in the serum is often calculated in medicine when attempting to identify the cause of metabolic acidosis...
is considered very useful. It is calculated by subtracting the chloride and bicarbonate levels from the sodium.
Anion gap = ( [Na+] ) - ( [Cl-]+[HCO3-] )
As sodium is the main extracellular cation, and chloride and bicarbonate are the main anions, the result should reflect the remaining anions. Normally, this concentration is about 8-16 mmol/l (12±4). An elevated anion gap (i.e. > 16 mmol/l) can indicate particular types of metabolic acidosis, particularly certain poisons, lactate acidosis and ketoacidosis.
As the differential diagnosis
Differential diagnosis
A differential diagnosis is a systematic diagnostic method used to identify the presence of an entity where multiple alternatives are possible , and may also refer to any of the included candidate alternatives A differential diagnosis (sometimes abbreviated DDx, ddx, DD, D/Dx, or ΔΔ) is a...
is made, certain other tests may be necessary, including toxicological screening and imaging of the kidneys. It is also important to differentiate between acidosis-induced hyperventilation and asthma; otherwise, treatment could lead to inappropriate bronchodilation.http://www.ncbi.nlm.nih.gov/sites/entrez/17906597?dopt=Abstract&holding=f1000,f1000m,isrctn
Causes
Metabolic acidosis occurs when the body produces too much acid, or when the kidneys are not removing enough acid from the body. There are several types of metabolic acidosis. The main causes are best grouped by their influence on the anion gapAnion gap
The anion gap is the difference in the measured cations and the measured anions in serum, plasma, or urine. The magnitude of this difference in the serum is often calculated in medicine when attempting to identify the cause of metabolic acidosis...
.
It bears noting that the anion gap can be spuriously normal in sampling errors of the sodium level, e.g. in extreme hypertriglyceridemia
Hypertriglyceridemia
In medicine, hypertriglyceridemia denotes high blood levels of triglycerides, the most abundant fatty molecule in most organisms. It has been associated with atherosclerosis, even in the absence of hypercholesterolemia . It can also lead to pancreatitis in excessive concentrations In medicine,...
. The anion gap can be increased due to relatively low levels of cations other than sodium and potassium (e.g. calcium or magnesium).
Increased anion gap
Causes include:- lactic acidosisLactic acidosisLactic acidosis is a physiological condition characterized by low pH in body tissues and blood accompanied by the buildup of lactate especially D-lactate, and is considered a distinct form of metabolic acidosis. The condition typically occurs when cells receive too little oxygen , for example...
- ketoacidosisKetoacidosisKetoacidosis is a metabolic state associated with high concentrations of ketone bodies, formed by the breakdown of fatty acids and the deamination of amino acids. The two common ketones produced in humans are acetoacetic acid and β-hydroxybutyrate....
- chronic renal failureChronic renal failureChronic kidney disease , also known as chronic renal disease, is a progressive loss in renal function over a period of months or years. The symptoms of worsening kidney function are unspecific, and might include feeling generally unwell and experiencing a reduced appetite...
(accumulation of sulfateSulfateIn inorganic chemistry, a sulfate is a salt of sulfuric acid.-Chemical properties:...
s, phosphatePhosphateA phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
s, ureaUreaUrea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
) - intoxication:
- organic acidOrganic acidAn organic acid is an organic compound with acidic properties. The most common organic acids are the carboxylic acids, whose acidity is associated with their carboxyl group –COOH. Sulfonic acids, containing the group –SO2OH, are relatively stronger acids. The relative stability of the conjugate...
s (salicylates, ethanolEthanolEthanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
, methanolMethanolMethanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
, formaldehydeFormaldehydeFormaldehyde is an organic compound with the formula CH2O. It is the simplest aldehyde, hence its systematic name methanal.Formaldehyde is a colorless gas with a characteristic pungent odor. It is an important precursor to many other chemical compounds, especially for polymers...
, ethylene glycolEthylene glycolEthylene glycol is an organic compound widely used as an automotive antifreeze and a precursor to polymers. In its pure form, it is an odorless, colorless, syrupy, sweet-tasting liquid...
, paraldehydeParaldehydeParaldehyde is the cyclic trimer of acetaldehyde molecules. Formally, it is a derivative of 1,3,5-trioxane. The corresponding tetramer is metaldehyde. A colourless liquid, it is sparingly soluble in water and highly soluble in alcohol. Paraldehyde slowly oxidizes in air, turning brown and producing...
, INH) - sulfates, metforminMetforminMetformin is an oral antidiabetic drug in the biguanide class. It is the first-line drug of choice for the treatment of type 2 diabetes, in particular, in overweight and obese people and those with normal kidney function. Its use in gestational diabetes has been limited by safety concerns...
(Glucophage)
- organic acid
- massive rhabdomyolysisRhabdomyolysisRhabdomyolysis is a condition in which damaged skeletal muscle tissue breaks down rapidly. Breakdown products of damaged muscle cells are released into the bloodstream; some of these, such as the protein myoglobin, are harmful to the kidneys and may lead to kidney failure...
Normal anion gap
Causes include:- longstanding diarrheaDiarrheaDiarrhea , also spelled diarrhoea, is the condition of having three or more loose or liquid bowel movements per day. It is a common cause of death in developing countries and the second most common cause of infant deaths worldwide. The loss of fluids through diarrhea can cause dehydration and...
(bicarbonate loss) - pancreatic fistulaPancreatic fistulaA pancreatic fistula is an abnormal communication between the pancreas and other organs due to leakage of pancreatic secretions from damaged pancreatic ducts. An external pancreatic fistula is one that communicates with the skin, and is also known as a pancreaticocutaneous fistula, whereas an...
- uretero-sigmoidostomy
- Renal tubular acidosisRenal tubular acidosisRenal tubular acidosis is a medical condition that involves an accumulation of acid in the body due to a failure of the kidneys to appropriately acidify the urine. When blood is filtered by the kidney, the filtrate passes through the tubules of the nephron, allowing for exchange of salts, acid...
(RTA) - intoxication:
- ammonium chlorideAmmonium chlorideAmmonium chloride NH4Cl is an inorganic compound with the formula NH4Cl. It is a white crystalline salt that is highly soluble in water. Solutions of ammonium chloride are mildly acidic. Sal ammoniac is a name of natural, mineralogical form of ammonium chloride...
- acetazolamideAcetazolamideAcetazolamide, sold under the trade name Diamox, is a carbonic anhydrase inhibitor that is used to treat glaucoma, epileptic seizures, Idiopathic intracranial hypertension , altitude sickness, cystinuria, and dural ectasia...
(Diamox) - bile acid sequestrantBile acid sequestrantThe bile acid sequestrants are a group of medications used to bind certain components of bile in the gastrointestinal tract. They disrupt the enterohepatic circulation of bile acids by sequestering them and preventing their reabsorption from the gut. In general, they are classified as hypolipidemic...
s - isopropyl alcoholIsopropyl alcoholIsopropyl alcohol is a common name for a chemical compound with the molecular formula C3H8O. It is a colorless, flammable chemical compound with a strong odor...
- ammonium chloride
- renal failureRenal failureRenal failure or kidney failure describes a medical condition in which the kidneys fail to adequately filter toxins and waste products from the blood...
(occasionally) - Glue sniffing
- tolueneTolueneToluene, formerly known as toluol, is a clear, water-insoluble liquid with the typical smell of paint thinners. It is a mono-substituted benzene derivative, i.e., one in which a single hydrogen atom from the benzene molecule has been replaced by a univalent group, in this case CH3.It is an aromatic...
Compensatory mechanisms
Metabolic acidosis is either due to increased generation of acid or an inability to generate sufficient bicarbonate. The body regulates the acidity of the blood by four buffering mechanisms.- bicarbonate buffering systemBicarbonate buffering systemThe bicarbonate buffering system is an important buffer system in the acid-base homeostasis of living things, including humans. As a buffer, it tends to maintain a relatively constant plasma pH and counteract any force that would alter it....
- IntracellularIntracellularNot to be confused with intercellular, meaning "between cells".In cell biology, molecular biology and related fields, the word intracellular means "inside the cell".It is used in contrast to extracellular...
buffering by absorption of hydrogen atoms by various molecules, including proteins, phosphates and carbonate in bone. - Respiratory compensationRespiratory compensationRespiratory compensation is a mechanism by which plasma pH can be altered by varying the respiratory rate. It is faster than renal compensation, but has less ability to restore normal values....
- Renal compensationRenal compensationRenal compensation is a mechanism by which the kidneys can regulate the plasma pH. It is slower than respiratory compensation, but has a greater ability to restore normal values....
Buffer
The decreased bicarbonate that distinguishes metabolic acidosis is therefore due to two separate processes: the buffer (from water and carbon dioxide) and additional renal generation. The buffer reactions are: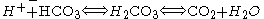
The Henderson-Hasselbalch equation
Henderson-Hasselbalch equation
In chemistry, the Henderson–Hasselbalch equation describes the derivation of pH as a measure of acidity in biological and chemical systems...
mathematically describes the relationship between blood pH and the components of the bicarbonate buffering system:
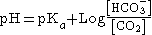
- Using Henry's Law, we can say that [CO2]=0.03xPaCO2
- (PaCO2 is the pressure of CO2 in arterial blood)
- Adding the other normal values, we get

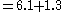
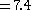
Treatment
A pH under 7.1 is an emergency, due to the risk of cardiac arrhythmias, and may warrant treatment with intravenous bicarbonate. Bicarbonate is given at 50-100 mmol at a time under scrupulous monitoring of the arterial blood gas readings. This intervention however, is not effective in case of lactic acidosisLactic acidosis
Lactic acidosis is a physiological condition characterized by low pH in body tissues and blood accompanied by the buildup of lactate especially D-lactate, and is considered a distinct form of metabolic acidosis. The condition typically occurs when cells receive too little oxygen , for example...
.
If the acidosis is particularly severe and/or there may be intoxication, consultation with the nephrology
Nephrology
Nephrology is a branch of internal medicine and pediatrics dealing with the study of the function and diseases of the kidney.-Scope of the specialty:...
team is considered useful, as dialysis
Dialysis
In medicine, dialysis is a process for removing waste and excess water from the blood, and is primarily used to provide an artificial replacement for lost kidney function in people with renal failure...
may clear both the intoxication and the acidosis.
See also
- Trauma triad of death
- Metabolic alkalosisMetabolic alkalosisMetabolic alkalosis is a metabolic condition in which the pH of tissue is elevated beyond the normal range . This is the result of decreased hydrogen ion concentration, leading to increased bicarbonate, or alternatively a direct result of increased bicarbonate concentrations.-Terminology:*Alkalosis...
- Respiratory acidosisRespiratory acidosisRespiratory acidosis is a medical condition in which decreased ventilation causes increased blood carbon dioxide concentration and decreased pH ....
- Respiratory alkalosisRespiratory alkalosisRespiratory alkalosis is a medical condition in which increased respiration elevates the blood pH...

