Methohexital
Encyclopedia
Methohexital, also called methohexitone, (marketed under the brand name Brevital) is a drug which is a barbiturate
derivative. It is classified as short-acting, and has a rapid onset of action. It is similar in its effects to sodium thiopental
, a drug with which it competed in the market for anaesthetics.
s at GABAA receptors. This increases the length of time which the Cl− ionopores are open, thus causing an inhibitory
effect.
Metabolism of methohexital is primarily hepatic (i.e., taking place in the liver) via demethylation and oxidation. Side-chain oxidation is the primary means of metabolism involvedtermination of the drug's biological activity.
Protein binding is approximately 73% for methohexital.
, and is generally provided as a sodium salt (i.e. methohexital sodium). It is only used in hospital
or similar settings, under strict supervision. It has been commonly used to induce deep sedation, "twilight sleep" or general anesthesia for oral surgery and dentistry. It is also used to induce anesthesia prior to ECT (electroconvulsive therapy).
. The resulting allyl-(1-methyl-2-pentynyl) malonic ester is synthesized by subsequent alkylation of the malonic ester itself, beginning with 2-bromo-3-hexyne, which gives (1-methyl-2-pentynyl)malonic ester, and then by allylbromide. In the final step, reaction of the disubstituted malonic ester with N-methylurea gives desired methohexital.
W.J. Doran, (1959).
Barbiturate
Barbiturates are drugs that act as central nervous system depressants, and can therefore produce a wide spectrum of effects, from mild sedation to total anesthesia. They are also effective as anxiolytics, as hypnotics, and as anticonvulsants...
derivative. It is classified as short-acting, and has a rapid onset of action. It is similar in its effects to sodium thiopental
Sodium thiopental
Sodium thiopental, better known as Sodium Pentothal , thiopental, thiopentone sodium, or Trapanal , is a rapid-onset short-acting barbiturate general anaesthetic...
, a drug with which it competed in the market for anaesthetics.
Pharmacology
Methohexital binds to a distinct site which is associated with Cl− ionophoreIonophore
An ionophore is a lipid-soluble molecule usually synthesized by microorganisms to transport ions across the lipid bilayer of the cell membrane...
s at GABAA receptors. This increases the length of time which the Cl− ionopores are open, thus causing an inhibitory
Inhibitory postsynaptic potential
An inhibitory postsynaptic potential is a synaptic potential that decreases the chance that a future action potential will occur in a postsynaptic neuron or α-motoneuron...
effect.
Metabolism of methohexital is primarily hepatic (i.e., taking place in the liver) via demethylation and oxidation. Side-chain oxidation is the primary means of metabolism involvedtermination of the drug's biological activity.
Protein binding is approximately 73% for methohexital.
Indications
Methohexital is primarily used to induce anesthesiaAnesthesia
Anesthesia, or anaesthesia , traditionally meant the condition of having sensation blocked or temporarily taken away...
, and is generally provided as a sodium salt (i.e. methohexital sodium). It is only used in hospital
Hospital
A hospital is a health care institution providing patient treatment by specialized staff and equipment. Hospitals often, but not always, provide for inpatient care or longer-term patient stays....
or similar settings, under strict supervision. It has been commonly used to induce deep sedation, "twilight sleep" or general anesthesia for oral surgery and dentistry. It is also used to induce anesthesia prior to ECT (electroconvulsive therapy).
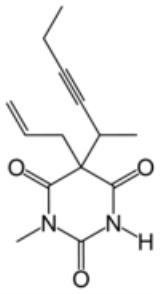
Chemistry
Methohexital, 5-allyl-1-methyl-5-(1-methyl-2-pentinyl barbituric acid, is synthesized in the classic manner of making barbituric acid derivatives, in particular by the reaction of malonic ester derivatives with derivatives of ureaUrea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
. The resulting allyl-(1-methyl-2-pentynyl) malonic ester is synthesized by subsequent alkylation of the malonic ester itself, beginning with 2-bromo-3-hexyne, which gives (1-methyl-2-pentynyl)malonic ester, and then by allylbromide. In the final step, reaction of the disubstituted malonic ester with N-methylurea gives desired methohexital.
W.J. Doran, (1959).

