
Methylene
Encyclopedia
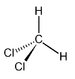

- the -CH2- (methanediyl) substituent group: e.g., dichloromethaneDichloromethaneDichloromethane is an organic compound with the formula CH2Cl2. This colorless, volatile liquid with a moderately sweet aroma is widely used as a solvent. Although it is not miscible with water, it is miscible with many organic solvents...
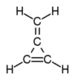
(also known as methylene chloride). - the =CH2 (methylidene) substituent group: e.g., methylenecyclopropeneMethylenecyclopropene3-Methylenecyclopropene is a hydrocarbon with chemical formula 44 and CAS number 4095-06-1. It dimerizes or polymerizes readily, but is more stable than cyclobutadiene....
. - the :CH2 molecule: A carbeneCarbeneIn chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
known as methyleneMethyleneMethylene is a chemical species in which a carbon atom is bonded to two hydrogen atoms. Three different possibilities present themselves:* the -CH2- substituent group: e.g., dichloromethane ....
. A carbene is a highly reactive organic molecule having a divalentDivalentIn chemistry, a divalent ion or molecule has a valence of two and thus can form two bonds with other ions or molecules. An older term for divalent is bivalent....
carbonCarbonCarbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
atom with six valenceValence (chemistry)In chemistry, valence, also known as valency or valence number, is a measure of the number of bonds formed by an atom of a given element. "Valence" can be defined as the number of valence bonds...
electrons.
Methylene groups in a chain or ring contribute to its size and lipophilicity. Methylene was also the original name for methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
.

