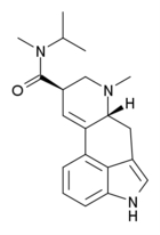
Methylisopropyllysergamide
Encyclopedia
Methylisopropyllysergamide (lysergic acid methylisopropyl amide, MIPLA) is an analogue of LSD
that was originally discovered by Albert Hofmann
at Sandoz
during the original structure-activity research into LSD. It has subsequently been investigated in more detail by the team led by David E. Nichols
at Purdue University
. Methylisopropyllysergamide is a structural isomer of LSD, with the alkyl groups on the amide nitrogen having been subjected to a methylene shuffle. MIPLA and its ethylisopropyl homologue are the only simple N,N-dialkyl lysergamides that approach the potency of LSD itself, being around 1/3-1/2 the potency of LSD, while all other dialkyl analogues tested (dimethyl, dipropyl, methylethyl etc.) are only around 1/10 as potent as LSD, although some N-monoalkyl lysergamides such as the sec-butyl
and t-butyl derivatives were also found to show an activity profile and potency comparable to LSD, and the mono-isopropyl derivative is only slightly weaker than MIPLA. Apart from its lower potency, the hallucinogenic effects of methylisopropyllysergamide are similar to those of LSD itself, and the main use for this drug has been in studies of the binding site at the 5-HT2A
receptor
through which LSD exerts most of its pharmacological effects.
LSD
Lysergic acid diethylamide, abbreviated LSD or LSD-25, also known as lysergide and colloquially as acid, is a semisynthetic psychedelic drug of the ergoline family, well known for its psychological effects which can include altered thinking processes, closed and open eye visuals, synaesthesia, an...
that was originally discovered by Albert Hofmann
Albert Hofmann
Albert Hofmann was a Swiss scientist known best for being the first person to synthesize, ingest and learn of the psychedelic effects of lysergic acid diethylamide . He authored more than 100 scientific articles and a number of books, including LSD: My Problem Child...
at Sandoz
Sandoz
Founded in 2003, Sandoz presently is the generic drug subsidiary of Novartis, a multinational pharmaceutical company. The company develops, manufactures and markets generic drugs as well as pharmaceutical and biotechnological active ingredients....
during the original structure-activity research into LSD. It has subsequently been investigated in more detail by the team led by David E. Nichols
David E. Nichols
David E. Nichols is an American pharmacologist and medicinal chemist.Presently the Robert C. and Charlotte P. Anderson Distinguished Chair in Pharmacology at Purdue University, Nichols has worked in the field of psychoactive drugs since 1969...
at Purdue University
Purdue University
Purdue University, located in West Lafayette, Indiana, U.S., is the flagship university of the six-campus Purdue University system. Purdue was founded on May 6, 1869, as a land-grant university when the Indiana General Assembly, taking advantage of the Morrill Act, accepted a donation of land and...
. Methylisopropyllysergamide is a structural isomer of LSD, with the alkyl groups on the amide nitrogen having been subjected to a methylene shuffle. MIPLA and its ethylisopropyl homologue are the only simple N,N-dialkyl lysergamides that approach the potency of LSD itself, being around 1/3-1/2 the potency of LSD, while all other dialkyl analogues tested (dimethyl, dipropyl, methylethyl etc.) are only around 1/10 as potent as LSD, although some N-monoalkyl lysergamides such as the sec-butyl
Lysergic acid 2-butyl amide
Lysergic acid 2-butyl amide is an analogue of LSD originally developed by Richard Pioch at Eli Lilly in the 1950s, but mostly publicised through research conducted by the team led by David E. Nichols at Purdue University...
and t-butyl derivatives were also found to show an activity profile and potency comparable to LSD, and the mono-isopropyl derivative is only slightly weaker than MIPLA. Apart from its lower potency, the hallucinogenic effects of methylisopropyllysergamide are similar to those of LSD itself, and the main use for this drug has been in studies of the binding site at the 5-HT2A
5-HT2A receptor
The mammalian 5-HT2A receptor is a subtype of the 5-HT2 receptor that belongs to the serotonin receptor family and is a G protein-coupled receptor . This is the main excitatory receptor subtype among the GPCRs for serotonin , although 5-HT2A may also have an inhibitory effect on certain areas such...
receptor
Receptor (biochemistry)
In biochemistry, a receptor is a molecule found on the surface of a cell, which receives specific chemical signals from neighbouring cells or the wider environment within an organism...
through which LSD exerts most of its pharmacological effects.

