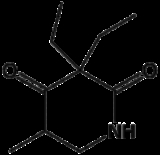
Methyprylon
Encyclopedia
Methyprylon is a sedative
of the piperidinedione
derivative family developed by Hoffmann-La Roche.
This medicine was used for treating insomnia
, but is now rarely used as it has been replaced by newer drugs with fewer side effects, such as benzodiazepines.
Methyprylon was withdrawn from the US market in June 1989 and the Canadian market in September 1990.
A case report found the pharmacokinetics of methyprylon nonlinear (concentration dependent) in an overdose case; explanations included saturation or inhibition of metabolic pathways. The generally accepted half-life for a therapeutic dose was not found appropriate in intoxicated patients and would underestimate the time required to reach a safe concentration of the drug.
pyrithyldione
by treating with ammonia and heating. The lactam is formylated
at postion-5 and reduced to yield methyprylon.
Sedative
A sedative or tranquilizer is a substance that induces sedation by reducing irritability or excitement....
of the piperidinedione
Piperidinedione
Piperidinediones are a derivatives of piperidine with two ketone functional groups. There are six isomers, each of which has a molecular weight of 113.115 and a formula of C5H7NO2...
derivative family developed by Hoffmann-La Roche.
This medicine was used for treating insomnia
Insomnia
Insomnia is most often defined by an individual's report of sleeping difficulties. While the term is sometimes used in sleep literature to describe a disorder demonstrated by polysomnographic evidence of disturbed sleep, insomnia is often defined as a positive response to either of two questions:...
, but is now rarely used as it has been replaced by newer drugs with fewer side effects, such as benzodiazepines.
Methyprylon was withdrawn from the US market in June 1989 and the Canadian market in September 1990.
Adverse effects
Side effects can include: Skin rash, fever, depression, ulcers or sores in mouth or throat, unusual bleeding or bruising, confusion, fast heartbeat, respiratory depression, swelling of feet or lower legs, dizziness, drowsiness, headache, double vision, clumsiness, constipation, diarrhea, nausea, vomiting, unusual weakness.Pharmacokinetics
A study of single oral doses of 300 mg in healthy volunteers found that the zero-order absorption model fit the data best. Mean (+/- SD) values for the half-life (9.2 +/- 2.2 h), apparent clearance, (11.91 +/- 4.42 mL/h/kg) and apparent steady-state volume of distribution, (0.97 +/- 0.33 L/kg) were found.A case report found the pharmacokinetics of methyprylon nonlinear (concentration dependent) in an overdose case; explanations included saturation or inhibition of metabolic pathways. The generally accepted half-life for a therapeutic dose was not found appropriate in intoxicated patients and would underestimate the time required to reach a safe concentration of the drug.
Synthesis
Methyprylon may be synthesized starting from an aldol condensation of ethyl 2,2-diethyl-3-oxobutanoate with ethyl formate. The resulting enol is converted to the lactamLactam
A lactam is a cyclic amide. Prefixes indicate how many carbon atoms are present in the ring: β-lactam , γ-lactam , δ-lactam...
pyrithyldione
Pyrithyldione
Pyrithyldione is a psychoactive drug invented in 1949.An improved method of manufacture was patented by Roche in 1959.It was used as a hypnotic or sedative and presumed to be less toxic than barbiturates....
by treating with ammonia and heating. The lactam is formylated
Formylation reaction
A formylation reaction in organic chemistry is the catch-all name for any organic reaction in which an organic compound is functionalized with a formyl group .Aromatic formylation reactions via electrophilic aromatic substitution include:...
at postion-5 and reduced to yield methyprylon.

