
Nernst-Planck equation
Encyclopedia
The Nernst–Planck equation is a conservation of mass equation used to describe the motion of chemical species in a fluid medium. It describes the flux of ion
s under the influence of both an ionic concentration gradient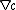 and an electric field
and an electric field
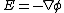 . It extends Fick's law of diffusion
. It extends Fick's law of diffusion
for the case where the diffusing particles are also moved with respect to the fluid by electrostatic forces.
The Nernst–Planck equation is given by:
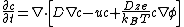
Where t is time, D is the diffusivity of the chemical species, c is the concentration of the species, and u is the velocity of the fluid, z is the valence of ionic species, e is the elementary charge
,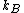 is the Boltzmann constant and T is the temperature.
is the Boltzmann constant and T is the temperature.
If the diffusing particles are themselves charged they influence the electric field on moving. Hence the Nernst–Planck equation is applied in describing the ion-exchange kinetics
in soils.
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
s under the influence of both an ionic concentration gradient
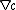 and an electric field
and an electric fieldElectric field
In physics, an electric field surrounds electrically charged particles and time-varying magnetic fields. The electric field depicts the force exerted on other electrically charged objects by the electrically charged particle the field is surrounding...
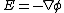 . It extends Fick's law of diffusion
. It extends Fick's law of diffusionFick's law of diffusion
Fick's laws of diffusion describe diffusion and can be used to solve for the diffusion coefficient, D. They were derived by Adolf Fick in the year 1855.- Fick's first law :...
for the case where the diffusing particles are also moved with respect to the fluid by electrostatic forces.
The Nernst–Planck equation is given by:
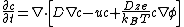
Where t is time, D is the diffusivity of the chemical species, c is the concentration of the species, and u is the velocity of the fluid, z is the valence of ionic species, e is the elementary charge
Elementary charge
The elementary charge, usually denoted as e, is the electric charge carried by a single proton, or equivalently, the absolute value of the electric charge carried by a single electron. This elementary charge is a fundamental physical constant. To avoid confusion over its sign, e is sometimes called...
,
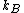 is the Boltzmann constant and T is the temperature.
is the Boltzmann constant and T is the temperature.If the diffusing particles are themselves charged they influence the electric field on moving. Hence the Nernst–Planck equation is applied in describing the ion-exchange kinetics
Chemical kinetics
Chemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
in soils.

