Nitrogen-vacancy center
Encyclopedia
The nitrogen-vacancy center (N-V center) is one of numerous point defect
s in diamond
. Its most explored and useful property is photoluminescence
, which can be easily detected from an individual N-V center. Electron spins at N-V centers, localized at atomic scales, can be manipulated at room temperature by applying a magnetic field
, electric field
, microwave
radiation or light, or a combination, resulting in sharp resonances in the intensity and wavelength of the photoluminescence. These resonances can be explained in terms of electron spin related phenomena such as quantum entanglement
, spin-orbit interaction
and Rabi oscillations
, and analysed using advanced quantum optics
theory. An individual N-V center can be viewed as a basic unit of a quantum computer
, and it has potential applications in novel, more efficient fields of electronics and computational science including spintronics
, quantum cryptography
and quantum computing
.
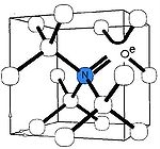
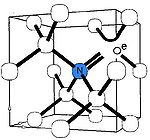 The nitrogen-vacancy center is a point defect
The nitrogen-vacancy center is a point defect
in the diamond lattice
. It consists of a nearest-neighbor pair of a nitrogen atom, which substitutes for a carbon atom, and a lattice vacancy.
Two charge states of this defect, neutral N-V0 and negative N-V−, are known from spectroscopic
studies using optical absorption, photoluminescence
(PL), electron paramagnetic resonance
(EPR) and optically detected magnetic resonance (ODMR), which can be viewed as a hybrid of PL and EPR; most details of the structure originate from EPR. A nitrogen atom has five valence electrons. Three of them covalently
bond to the carbon atoms and two remain non-bonded and are called a lone pair
. The vacancy has three unpaired electrons. Two of them make a quasi covalent bond and one remains unpaired. The overall symmetry, however, is axial (trigonal C3V); one can visualize this by imagining the three unpaired vacancy electrons continuously exchanging their roles.
The N-V0 thus has one unpaired electron and is paramagnetic. However, despite extensive efforts, electron paramagnetic resonance
signals from N-V0 avoided detection for decades until 2008. Optical excitation is required to bring the N-V0 defect into the EPR-detectable excited state; the signals from the ground state are presumably too broad for EPR detection.
In the negative charge state N-V−, the extra electron is located at the vacancy site forming a spin S=1 pair with one of the vacancy electrons. As in N-V0, the vacancy electrons are "exchanging roles" preserving the overall trigonal symmetry. This N-V− state is what is commonly, and somewhat incorrectly, called "the nitrogen-vacancy center". The neutral state has not yet been explored for spin manipulations.
The N-V centers are randomly oriented within a diamond crystal. Ion implantation
techniques can enable their artificial creation in predetermined positions.
Diamonds are notorious by having relatively large lattice strain. Strain splits and shifts optical transitions from individual centers resulting in broad lines in the ensembles of centers. Special care is taken to produce extremely sharp N-V lines (line width ~10 MHz) required for most experiments: high-quality, pure natural or better synthetic diamonds (type IIa) are selected. Many of them already have sufficient concentrations of grown-in N-V centers and are suitable for applications. If not, they are irradiated by high-energy particles and annealed. Selection of a certain irradiation dose allows tuning the concentration of produced N-V centers such that individual N-V centers are separated by micrometre-large distances. Then, individual N-V centers can be studied with standard optical microscope
s or, better, near-field scanning optical microscope
s having sub-micrometre resolution.
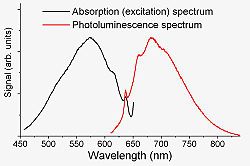 N-V− centers emit bright red light which can be conveniently excited by visible light sources, such as argon or krypton lasers
N-V− centers emit bright red light which can be conveniently excited by visible light sources, such as argon or krypton lasers
, frequency doubled Nd:YAG laser
s, dye laser
s, or He-Ne lasers. Laser illumination, however, also converts some N-V− into N-V0 centers. Emission is very quick (relaxation time ~10 ns). At room temperature, no sharp peaks are observed because of the thermal broadening. However, cooling the N-V− centers with liquid nitrogen
or liquid helium
dramatically narrows the lines down to few megahertz width.
An important property of the luminescence from individual N-V− centers is its high temporal stability. Whereas many single-molecular emitters bleach after emission of 106–108 photons, no bleaching is observed for the N-V centers at room temperature.
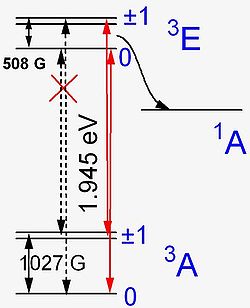 The energy level structure of the N-V− center was established by combining optical, electron paramagnetic resonance and theoretical results, as shown in the figure. In particular, several theoretical works have been done, using the Linear Combination of Atomic Orbitals (LCAO) approach, to build the electronic orbitals to describe the possible quantum states, looking at the NV center as a molecule. Moreover, group theory results are used, to take into account the symmetry of the diamond crystal, and so the symmetry of the NV itself. The energy levels are labeled according to the group theory, and in particular are labelled after the irreducible representations of the C3V symmetry group of the defect center, A1, A2 and E.
The energy level structure of the N-V− center was established by combining optical, electron paramagnetic resonance and theoretical results, as shown in the figure. In particular, several theoretical works have been done, using the Linear Combination of Atomic Orbitals (LCAO) approach, to build the electronic orbitals to describe the possible quantum states, looking at the NV center as a molecule. Moreover, group theory results are used, to take into account the symmetry of the diamond crystal, and so the symmetry of the NV itself. The energy levels are labeled according to the group theory, and in particular are labelled after the irreducible representations of the C3V symmetry group of the defect center, A1, A2 and E.
The numbers 3 in 3A and 1 in 1A represent the number of allowable ms spin states, or the spin multiplicity, which range from –S to S for a total of 2S+1 possible states. If S = 1, ms can be –1, 0, or 1. The 1A level is predicted by theory but not directly observed in experiment, and it is believed to play an important role in the quenching of photoluminescence.
In the absence of an external magnetic field, the ground and excited states are split by the magnetic interaction between the two unpaired electrons at the N-V− center (see microscopic model): when two electrons have parallel spins (ms=±1), their energy is higher than when spins are antiparallel (ms=0). The farther apart the electrons are, the weaker their interaction energy D (roughly D ~1/r3). Thus the smaller splitting in the excited state can be viewed in terms of larger electron-electron separation in the excited state. When an external magnetic field is applied to the N-V− center, it does not affect the ms=0 states nor the 1A state (because it has S = 0), but it splits the ms = ±1 levels. If a magnetic field is oriented along the defect axis and reaches about 1027 G (or 508 G) then the ms = –1 and ms = 0 states in the ground (or excited) state become equal in energy; they strongly interact resulting in so-called spin polarization
, which strongly affects the intensity of optical absorption and luminescence transitions involving those states.
In order to understand why this happens, we have to keep in mind that transitions between electronic states are mediated by a photon
which cannot change overall spin
. Thus optical transitions must preserve the total spin and occur between levels of the same total spin. For this reason, transitions 3E↔1A and 1A ↔ 3A are non-radiative and quench the luminescence. Whereas ms = −1 (excited state) ↔ ms = 0 (ground state) transition was forbidden in the absence of an external magnetic field, it becomes allowed when a magnetic field mixes the ms = −1 and ms = 0 levels in the ground state. As a measurable outcome of this phenomenon, luminescence intensity can be strongly modulated by magnetic field.
There is an additional level splitting in the excited 3E state due to the orbital degeneracy and spin-orbit interaction
. Importantly, this splitting can be modulated by applying a static electric field
, in a similar fashion to the magnetic field mechanism outlined above, though the physics of the splitting is somewhat more complex. Nevertheless, an important practical outcome is that the intensity and position of the luminescence lines can be modulated by applying electric or/and magnetic fields.
The energy difference between the ms = 0 and ms = ±1 states corresponds to the microwave
region. Thus by irradiating the N-V centers with microwave radiation, one can change the relative population of those levels, thereby again modulating the luminescence intensity.
There is an additional splitting of the ms = ±1 energy levels, which originates from the "hyperfine
" interaction between the nuclear and electron spins. Thus finally, the optical absorption and luminescence from the N-V− center consists of roughly a dozen sharp lines with a separation in the MHz-GHz range, and all those lines can be resolved, given proper sample preparation. The intensity and position of those lines can be modulated using the following tools:
As a final remark, it should be noted that the above-described energy structure is by no means exceptional for a defect in diamond or other semiconductor (see, e.g.). It was not this structure alone, but a combination of several favorable factors (previous knowledge, easy production and excitation, etc.) which suggested the use of the N-V− center.
.
Regarding the characterization of single N-V− centers, this field is very competitive nowadays; numerous papers have been published in the major scientific journals and reviewing them is hardly possible on these pages. However, one result published in 1997 is worth mentioning. In that paper, it was demonstrated that the fluorescence of single N-V− centers can be detected by room-temperature fluorescence microscopy and also that the defect shows perfect photostability. Also one of the outstanding properties of the NV center was demonstrated, namely room temperature optically detected magnetic resonance.
Crystallographic defects in diamond
Imperfections in the crystal lattice of diamond are common. Such crystallographic defects in diamond may be the result of lattice irregularities or extrinsic substitutional or interstitial impurities, introduced during or after the diamond growth...
s in diamond
Diamond
In mineralogy, diamond is an allotrope of carbon, where the carbon atoms are arranged in a variation of the face-centered cubic crystal structure called a diamond lattice. Diamond is less stable than graphite, but the conversion rate from diamond to graphite is negligible at ambient conditions...
. Its most explored and useful property is photoluminescence
Photoluminescence
Photoluminescence is a process in which a substance absorbs photons and then re-radiates photons. Quantum mechanically, this can be described as an excitation to a higher energy state and then a return to a lower energy state accompanied by the emission of a photon...
, which can be easily detected from an individual N-V center. Electron spins at N-V centers, localized at atomic scales, can be manipulated at room temperature by applying a magnetic field
Magnetic field
A magnetic field is a mathematical description of the magnetic influence of electric currents and magnetic materials. The magnetic field at any given point is specified by both a direction and a magnitude ; as such it is a vector field.Technically, a magnetic field is a pseudo vector;...
, electric field
Electric field
In physics, an electric field surrounds electrically charged particles and time-varying magnetic fields. The electric field depicts the force exerted on other electrically charged objects by the electrically charged particle the field is surrounding...
, microwave
Microwave
Microwaves, a subset of radio waves, have wavelengths ranging from as long as one meter to as short as one millimeter, or equivalently, with frequencies between 300 MHz and 300 GHz. This broad definition includes both UHF and EHF , and various sources use different boundaries...
radiation or light, or a combination, resulting in sharp resonances in the intensity and wavelength of the photoluminescence. These resonances can be explained in terms of electron spin related phenomena such as quantum entanglement
Quantum entanglement
Quantum entanglement occurs when electrons, molecules even as large as "buckyballs", photons, etc., interact physically and then become separated; the type of interaction is such that each resulting member of a pair is properly described by the same quantum mechanical description , which is...
, spin-orbit interaction
Spin-orbit interaction
In quantum physics, the spin-orbit interaction is any interaction of a particle's spin with its motion. The first and best known example of this is that spin-orbit interaction causes shifts in an electron's atomic energy levels due to electromagnetic interaction between the electron's spin and...
and Rabi oscillations
Rabi cycle
In physics, the Rabi cycle is the cyclic behaviour of a two-state quantum system in the presence of an oscillatory driving field. A two-state system has two possible states, and if they are not degenerate energy levels the system can become "excited" when it absorbs a quantum of energy.The effect...
, and analysed using advanced quantum optics
Quantum optics
Quantum optics is a field of research in physics, dealing with the application of quantum mechanics to phenomena involving light and its interactions with matter.- History of quantum optics :...
theory. An individual N-V center can be viewed as a basic unit of a quantum computer
Quantum computer
A quantum computer is a device for computation that makes direct use of quantum mechanical phenomena, such as superposition and entanglement, to perform operations on data. Quantum computers are different from traditional computers based on transistors...
, and it has potential applications in novel, more efficient fields of electronics and computational science including spintronics
Spintronics
Spintronics , also known as magnetoelectronics, is an emerging technology that exploits both the intrinsic spin of the electron and its associated magnetic moment, in addition to its fundamental electronic charge, in solid-state devices.An additional effect occurs when a spin-polarized current is...
, quantum cryptography
Quantum cryptography
Quantum key distribution uses quantum mechanics to guarantee secure communication. It enables two parties to produce a shared random secret key known only to them, which can then be used to encrypt and decrypt messages...
and quantum computing
Quantum computer
A quantum computer is a device for computation that makes direct use of quantum mechanical phenomena, such as superposition and entanglement, to perform operations on data. Quantum computers are different from traditional computers based on transistors...
.
Structure

Crystallographic defects in diamond
Imperfections in the crystal lattice of diamond are common. Such crystallographic defects in diamond may be the result of lattice irregularities or extrinsic substitutional or interstitial impurities, introduced during or after the diamond growth...
in the diamond lattice
Diamond cubic
The diamond cubic crystal structure is a repeating pattern of 8 atoms that certain materials may adopt as they solidify. While the first known example was diamond, other elements in group IV also adopt this structure, including tin, the semiconductors silicon and germanium, and silicon/germanium...
. It consists of a nearest-neighbor pair of a nitrogen atom, which substitutes for a carbon atom, and a lattice vacancy.
Two charge states of this defect, neutral N-V0 and negative N-V−, are known from spectroscopic
Spectroscopy
Spectroscopy is the study of the interaction between matter and radiated energy. Historically, spectroscopy originated through the study of visible light dispersed according to its wavelength, e.g., by a prism. Later the concept was expanded greatly to comprise any interaction with radiative...
studies using optical absorption, photoluminescence
Photoluminescence
Photoluminescence is a process in which a substance absorbs photons and then re-radiates photons. Quantum mechanically, this can be described as an excitation to a higher energy state and then a return to a lower energy state accompanied by the emission of a photon...
(PL), electron paramagnetic resonance
Electron paramagnetic resonance
Electron paramagnetic resonance or electron spin resonance spectroscopyis a technique for studying chemical species that have one or more unpaired electrons, such as organic and inorganic free radicals or inorganic complexes possessing a transition metal ion...
(EPR) and optically detected magnetic resonance (ODMR), which can be viewed as a hybrid of PL and EPR; most details of the structure originate from EPR. A nitrogen atom has five valence electrons. Three of them covalently
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
bond to the carbon atoms and two remain non-bonded and are called a lone pair
Lone pair
In chemistry, a lone pair is a valence electron pair without bonding or sharing with other atoms. They are found in the outermost electron shell of an atom, so lone pairs are a subset of a molecule's valence electrons...
. The vacancy has three unpaired electrons. Two of them make a quasi covalent bond and one remains unpaired. The overall symmetry, however, is axial (trigonal C3V); one can visualize this by imagining the three unpaired vacancy electrons continuously exchanging their roles.
The N-V0 thus has one unpaired electron and is paramagnetic. However, despite extensive efforts, electron paramagnetic resonance
Electron paramagnetic resonance
Electron paramagnetic resonance or electron spin resonance spectroscopyis a technique for studying chemical species that have one or more unpaired electrons, such as organic and inorganic free radicals or inorganic complexes possessing a transition metal ion...
signals from N-V0 avoided detection for decades until 2008. Optical excitation is required to bring the N-V0 defect into the EPR-detectable excited state; the signals from the ground state are presumably too broad for EPR detection.
In the negative charge state N-V−, the extra electron is located at the vacancy site forming a spin S=1 pair with one of the vacancy electrons. As in N-V0, the vacancy electrons are "exchanging roles" preserving the overall trigonal symmetry. This N-V− state is what is commonly, and somewhat incorrectly, called "the nitrogen-vacancy center". The neutral state has not yet been explored for spin manipulations.
The N-V centers are randomly oriented within a diamond crystal. Ion implantation
Ion implantation
Ion implantation is a materials engineering process by which ions of a material are accelerated in an electrical field and impacted into another solid. This process is used to change the physical, chemical, or electrical properties of the solid...
techniques can enable their artificial creation in predetermined positions.
Production
Nitrogen-vacancy centers are produced from single substitutional nitrogen centers (called C or P1 centers in diamond literature) by irradiation followed by annealing at temperatures above 700 0C. A wide range of high-energy particles are suitable for such irradiation, including electrons, protons, neutrons, ions, and gamma photons. Irradiation produces lattice vacancies, which are a part of N-V centers. Those vacancies are immobile at room temperature, and annealing is required to move them. Single substitutional nitrogen produces strain in the diamond lattice; it therefore efficiently captures moving vacancies, producing the N-V centers.Diamonds are notorious by having relatively large lattice strain. Strain splits and shifts optical transitions from individual centers resulting in broad lines in the ensembles of centers. Special care is taken to produce extremely sharp N-V lines (line width ~10 MHz) required for most experiments: high-quality, pure natural or better synthetic diamonds (type IIa) are selected. Many of them already have sufficient concentrations of grown-in N-V centers and are suitable for applications. If not, they are irradiated by high-energy particles and annealed. Selection of a certain irradiation dose allows tuning the concentration of produced N-V centers such that individual N-V centers are separated by micrometre-large distances. Then, individual N-V centers can be studied with standard optical microscope
Optical microscope
The optical microscope, often referred to as the "light microscope", is a type of microscope which uses visible light and a system of lenses to magnify images of small samples. Optical microscopes are the oldest design of microscope and were possibly designed in their present compound form in the...
s or, better, near-field scanning optical microscope
Near-field scanning optical microscope
Near-field scanning optical microscopy is a microscopic technique for nanostructure investigation that breaks the far field resolution limit by exploiting the properties of evanescent waves. This is done by placing the detector very close to the specimen surface...
s having sub-micrometre resolution.
Basic optical properties

Ion laser
An ion laser is a gas laser which uses an ionized gas as its lasing medium.Like other gas lasers, ion lasers feature a sealed cavity containing the laser medium and mirrors forming a Fabry–Pérot resonator. Unlike HeNe lasers, the energy level transitions that contribute to laser action come from ions...
, frequency doubled Nd:YAG laser
Nd:YAG laser
Nd:YAG is a crystal that is used as a lasing medium for solid-state lasers. The dopant, triply ionized neodymium, typically replaces yttrium in the crystal structure of the yttrium aluminium garnet , since they are of similar size...
s, dye laser
Dye laser
A dye laser is a laser which uses an organic dye as the lasing medium, usually as a liquid solution. Compared to gases and most solid state lasing media, a dye can usually be used for a much wider range of wavelengths. The wide bandwidth makes them particularly suitable for tunable lasers and...
s, or He-Ne lasers. Laser illumination, however, also converts some N-V− into N-V0 centers. Emission is very quick (relaxation time ~10 ns). At room temperature, no sharp peaks are observed because of the thermal broadening. However, cooling the N-V− centers with liquid nitrogen
Liquid nitrogen
Liquid nitrogen is nitrogen in a liquid state at a very low temperature. It is produced industrially by fractional distillation of liquid air. Liquid nitrogen is a colourless clear liquid with density of 0.807 g/mL at its boiling point and a dielectric constant of 1.4...
or liquid helium
Liquid helium
Helium exists in liquid form only at extremely low temperatures. The boiling point and critical point depend on the isotope of the helium; see the table below for values. The density of liquid helium-4 at its boiling point and 1 atmosphere is approximately 0.125 g/mL Helium-4 was first liquefied...
dramatically narrows the lines down to few megahertz width.
An important property of the luminescence from individual N-V− centers is its high temporal stability. Whereas many single-molecular emitters bleach after emission of 106–108 photons, no bleaching is observed for the N-V centers at room temperature.
Energy level structure and its manipulation by external fields

The numbers 3 in 3A and 1 in 1A represent the number of allowable ms spin states, or the spin multiplicity, which range from –S to S for a total of 2S+1 possible states. If S = 1, ms can be –1, 0, or 1. The 1A level is predicted by theory but not directly observed in experiment, and it is believed to play an important role in the quenching of photoluminescence.
In the absence of an external magnetic field, the ground and excited states are split by the magnetic interaction between the two unpaired electrons at the N-V− center (see microscopic model): when two electrons have parallel spins (ms=±1), their energy is higher than when spins are antiparallel (ms=0). The farther apart the electrons are, the weaker their interaction energy D (roughly D ~1/r3). Thus the smaller splitting in the excited state can be viewed in terms of larger electron-electron separation in the excited state. When an external magnetic field is applied to the N-V− center, it does not affect the ms=0 states nor the 1A state (because it has S = 0), but it splits the ms = ±1 levels. If a magnetic field is oriented along the defect axis and reaches about 1027 G (or 508 G) then the ms = –1 and ms = 0 states in the ground (or excited) state become equal in energy; they strongly interact resulting in so-called spin polarization
Spin polarization
Spin polarization is the degree to which the spin, i.e., the intrinsic angular momentum of elementary particles, is aligned with a given direction. This property may pertain to the spin, hence to the magnetic moment, of conduction electrons in ferromagnetic metals, such as iron, giving rise to...
, which strongly affects the intensity of optical absorption and luminescence transitions involving those states.
In order to understand why this happens, we have to keep in mind that transitions between electronic states are mediated by a photon
Photon
In physics, a photon is an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation. It is also the force carrier for the electromagnetic force...
which cannot change overall spin
Spin (physics)
In quantum mechanics and particle physics, spin is a fundamental characteristic property of elementary particles, composite particles , and atomic nuclei.It is worth noting that the intrinsic property of subatomic particles called spin and discussed in this article, is related in some small ways,...
. Thus optical transitions must preserve the total spin and occur between levels of the same total spin. For this reason, transitions 3E↔1A and 1A ↔ 3A are non-radiative and quench the luminescence. Whereas ms = −1 (excited state) ↔ ms = 0 (ground state) transition was forbidden in the absence of an external magnetic field, it becomes allowed when a magnetic field mixes the ms = −1 and ms = 0 levels in the ground state. As a measurable outcome of this phenomenon, luminescence intensity can be strongly modulated by magnetic field.
There is an additional level splitting in the excited 3E state due to the orbital degeneracy and spin-orbit interaction
Spin-orbit interaction
In quantum physics, the spin-orbit interaction is any interaction of a particle's spin with its motion. The first and best known example of this is that spin-orbit interaction causes shifts in an electron's atomic energy levels due to electromagnetic interaction between the electron's spin and...
. Importantly, this splitting can be modulated by applying a static electric field
Electric field
In physics, an electric field surrounds electrically charged particles and time-varying magnetic fields. The electric field depicts the force exerted on other electrically charged objects by the electrically charged particle the field is surrounding...
, in a similar fashion to the magnetic field mechanism outlined above, though the physics of the splitting is somewhat more complex. Nevertheless, an important practical outcome is that the intensity and position of the luminescence lines can be modulated by applying electric or/and magnetic fields.
The energy difference between the ms = 0 and ms = ±1 states corresponds to the microwave
Microwave
Microwaves, a subset of radio waves, have wavelengths ranging from as long as one meter to as short as one millimeter, or equivalently, with frequencies between 300 MHz and 300 GHz. This broad definition includes both UHF and EHF , and various sources use different boundaries...
region. Thus by irradiating the N-V centers with microwave radiation, one can change the relative population of those levels, thereby again modulating the luminescence intensity.
There is an additional splitting of the ms = ±1 energy levels, which originates from the "hyperfine
Hyperfine structure
The term hyperfine structure refers to a collection of different effects leading to small shifts and splittings in the energy levels of atoms, molecules and ions. The name is a reference to the fine structure which results from the interaction between the magnetic moments associated with electron...
" interaction between the nuclear and electron spins. Thus finally, the optical absorption and luminescence from the N-V− center consists of roughly a dozen sharp lines with a separation in the MHz-GHz range, and all those lines can be resolved, given proper sample preparation. The intensity and position of those lines can be modulated using the following tools:
- Amplitude and orientation of magnetic fieldMagnetic fieldA magnetic field is a mathematical description of the magnetic influence of electric currents and magnetic materials. The magnetic field at any given point is specified by both a direction and a magnitude ; as such it is a vector field.Technically, a magnetic field is a pseudo vector;...
, which splits the ms = ±1 levels in the ground and excited states. - Amplitude and orientation of elastic fieldDeformation (mechanics)Deformation in continuum mechanics is the transformation of a body from a reference configuration to a current configuration. A configuration is a set containing the positions of all particles of the body...
(strain), which can be applied by, e.g., squeezing the diamond. Similar effects can be induced by applying electric fieldElectric fieldIn physics, an electric field surrounds electrically charged particles and time-varying magnetic fields. The electric field depicts the force exerted on other electrically charged objects by the electrically charged particle the field is surrounding...
, and the electric field can be controlled with much higher precision. - Continuous-wave microwaveMicrowaveMicrowaves, a subset of radio waves, have wavelengths ranging from as long as one meter to as short as one millimeter, or equivalently, with frequencies between 300 MHz and 300 GHz. This broad definition includes both UHF and EHF , and various sources use different boundaries...
radiation, which changes the population of the sublevels within the ground and excited state. - Tunable laserTunable laserA tunable laser is a laser whose wavelength of operation can be altered in a controlled manner. While all laser gain media allow small shifts in output wavelength, only a few types of lasers allow continuous tuning over a significant wavelength range....
, which can selectively excite certain sublevels of the ground and excited state. - In addition to those static perturbations, numerous dynamic effects (spin echoSpin echoIn magnetic resonance, a spin echo is the refocusing of precessing spin magnetisation by a pulse of resonant radiation. Modern nuclear magnetic resonance and magnetic resonance imaging rely heavily on this effect....
, Rabi oscillationsRabi cycleIn physics, the Rabi cycle is the cyclic behaviour of a two-state quantum system in the presence of an oscillatory driving field. A two-state system has two possible states, and if they are not degenerate energy levels the system can become "excited" when it absorbs a quantum of energy.The effect...
, etc.) can be exploited by applying a carefully designed sequence of microwave pulses. The first pulse coherently excites the electron spins, and this coherence is then manipulated and probed by the subsequent pulses. Those dynamic effects are rather important for practical realization of quantum computerQuantum computerA quantum computer is a device for computation that makes direct use of quantum mechanical phenomena, such as superposition and entanglement, to perform operations on data. Quantum computers are different from traditional computers based on transistors...
s, which ought to work at high frequency.
As a final remark, it should be noted that the above-described energy structure is by no means exceptional for a defect in diamond or other semiconductor (see, e.g.). It was not this structure alone, but a combination of several favorable factors (previous knowledge, easy production and excitation, etc.) which suggested the use of the N-V− center.
Biological applications
In addition to the quantum optical applications, luminescence from the N-V− centers can be applied for imaging biological processes, such as fluid flow in living cells. This application relies on good compatibility of diamond nanoparticles with the living cells and on favorable properties of photoluminescence from the N-V− centers (strong intensity, easy excitation and detection, temporal stability, etc.). Compared with large single-crystal diamonds, nanodiamonds are cheap (~1 USD per gram) and available from various suppliers. N-V− centers are produced in diamond powders with submicrometre particle size using the standard process of irradiation and annealing described above. Those nanodiamonds are introduced in a cell, and their luminescence is monitored using a standard fluorescence microscopeFluorescence microscope
A fluorescence microscope is an optical microscope used to study properties of organic or inorganic substances using the phenomena of fluorescence and phosphorescence instead of, or in addition to, reflection and absorption...
.
Historical remarks
The microscopic model and most optical properties of ensembles of the N-V− centers have been firmly established in the 1970s based on the optical measurements combined with uniaxial stress and on the electron paramagnetic resonance. However, a minor error in EPR results (it was assumed that illumination is required to observe N-V− EPR signals) resulted in the incorrect multiplicity assignments in the energy level structure. In 1991 it was shown that EPR can be observed without illumination, which established the energy level scheme shown above. The magnetic splitting in the excited state has been measured only recently.Regarding the characterization of single N-V− centers, this field is very competitive nowadays; numerous papers have been published in the major scientific journals and reviewing them is hardly possible on these pages. However, one result published in 1997 is worth mentioning. In that paper, it was demonstrated that the fluorescence of single N-V− centers can be detected by room-temperature fluorescence microscopy and also that the defect shows perfect photostability. Also one of the outstanding properties of the NV center was demonstrated, namely room temperature optically detected magnetic resonance.
See also
- Crystallographic defects in diamondCrystallographic defects in diamondImperfections in the crystal lattice of diamond are common. Such crystallographic defects in diamond may be the result of lattice irregularities or extrinsic substitutional or interstitial impurities, introduced during or after the diamond growth...
- Crystallographic defectCrystallographic defectCrystalline solids exhibit a periodic crystal structure. The positions of atoms or molecules occur on repeating fixed distances, determined by the unit cell parameters. However, the arrangement of atom or molecules in most crystalline materials is not perfect...
- Material properties of diamondMaterial properties of diamondDiamond is the allotrope of carbon in which the carbon atoms are arranged in the specific type of cubic lattice called diamond cubic. Diamond is an optically isotropic crystal that is transparent to opaque. Owing to its strong covalent bonding, diamond is the hardest naturally occurring material...

