
Non-stoichiometric compound
Encyclopedia
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
s with an elemental
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
composition that cannot be represented by a ratio of well-defined natural number
Natural number
In mathematics, the natural numbers are the ordinary whole numbers used for counting and ordering . These purposes are related to the linguistic notions of cardinal and ordinal numbers, respectively...
s, and therefore violate the law of definite proportions
Law of definite proportions
In chemistry, the law of definite proportions, sometimes called Proust's Law, states that a chemical compound always contains exactly the same proportion of elements by mass. An equivalent statement is the law of constant composition, which states that all samples of a given chemical compound have...
. Often, they are solid
Solid
Solid is one of the three classical states of matter . It is characterized by structural rigidity and resistance to changes of shape or volume. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire volume available to it like a...
s that contain crystallographic point defects
Crystallographic defect
Crystalline solids exhibit a periodic crystal structure. The positions of atoms or molecules occur on repeating fixed distances, determined by the unit cell parameters. However, the arrangement of atom or molecules in most crystalline materials is not perfect...
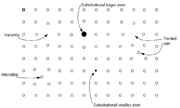
, such as interstitial atoms and vacancies, which result in excess or deficiency of an element, respectively. Since solids are overall electrically neutral, the defect in an ionic compound
Ionic compound
In chemistry, an ionic compound is a chemical compound in which ions are held together in a lattice structure by ionic bonds. Usually, the positively charged portion consists of metal cations and the negatively charged portion is an anion or polyatomic ion. Ions in ionic compounds are held together...
is compensated by a change in the charge of other atoms in the solid, either by changing their oxidation state, or by replacing them with atoms of different elements with a different charge.
Nonstoichiometry is pervasive for transition metal oxides
Transition metal oxides
Transition metal oxides comprise a class of materials that contain transition elements and oxygen. They include insulators as well as metals. Often the same material may display both types of transport properties, hence a Metal-Insulator transition, obtained by varying either temperature or...
, especially when the metal is not in its highest oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
. For example, although wüstite
Wüstite
Wüstite is a mineral form of iron oxide found with meteorites and native iron. It has a gray color with a greenish tint in reflected light. Wüstite crystallizes in the isometric - hexoctahedral crystal system in opaque to translucent metallic grains. It has a Mohs hardness of 5 to 5.5 and a...
(ferrous oxide
Iron(II) oxide
Iron oxide, also known as ferrous oxide, is one of the iron oxides. It is a black-colored powder with the chemical formula . It consists of the chemical element iron in the oxidation state of 2 bonded to oxygen. Its mineral form is known as wüstite. Iron oxide should not be confused with rust,...
) has an ideal (stoichiometric) formula FeO, the actual stoichiometry is closer to Fe0.95O. The non-stoichiometry occurs because of the ease of oxidation of Fe2+ to Fe3+ effectively replacing a small portion of Fe2+ with two thirds their number of Fe3+. Thus for every three "missing" Fe2+ ions, the crystal contains two Fe3+ ions to balance the charge. The composition of a non-stoichiometric compound usually varies in a continuous manner over a narrow range. Thus, the formula for wüstite is written as Fe1-xO, where x is a small number (0.05 in the previous example) representing the deviation from the "ideal" formula. Nonstoichiometry is especially important in solid, three-dimensional polymers that can tolerate mistakes. To some extent, entropy drives all solids to be non-stoichiometric. But for practical purposes, the term describes materials where the non-stoichiometry is measurable, usually at least 1% of the ideal composition.
Non-stoichiometric compounds are also known as berthollides (as opposed to the stoichiometric compounds or daltonides). The names come from Claude Louis Berthollet
Claude Louis Berthollet
Claude Louis Berthollet was a Savoyard-French chemist who became vice president of the French Senate in 1804.-Biography:...
and John Dalton
John Dalton
John Dalton FRS was an English chemist, meteorologist and physicist. He is best known for his pioneering work in the development of modern atomic theory, and his research into colour blindness .-Early life:John Dalton was born into a Quaker family at Eaglesfield, near Cockermouth, Cumberland,...
, respectively, who in the 19th century advocated rival theories of the composition of substances. Although Dalton "won" for the most part, it was later recognized that the law of definite proportions did have important exceptions.
Defects vs non-stoichiometry
The cuprate superconductors highlight the concept of "defect" structures, which is related to non-stoichiometry. YBa2Cu3O7−x can be viewed as a variant of the perovskite family of materials, which have idealized stoichiometry ABO3. For the cuprates, Y + Ba occupy "A sites" whereas Cu occupies the "B sites". The non-defect material would have the stoichiometry YBa2Cu3O9. Using this way of describing a structure, W40O118 is said to be a defect variant of WO3.Cuprates
Many non-stoichiometric compounds are important in solid state chemistry, and have applications in ceramicCeramic
A ceramic is an inorganic, nonmetallic solid prepared by the action of heat and subsequent cooling. Ceramic materials may have a crystalline or partly crystalline structure, or may be amorphous...
s and as superconductors. For example, yttrium barium copper oxide
Yttrium barium copper oxide
Yttrium barium copper oxide, often abbreviated YBCO, is a crystalline chemical compound with the formula YBa2Cu3O7. This material, a famous "high-temperature superconductor", achieved prominence because it was the first material to achieve superconductivity above the boiling point of liquid...
, arguably the most notable high-temperature superconductor, is a non-stoichiometric solid with a formula represented by YBa2Cu3O7−x. The critical temperature of the superconductor depends on the exact value of x. The stoichiometric species has x = 0, but this value can be as great as 1.
Tungsten oxides
It is sometimes difficult to determine if a material is non-stoichiometric or if the formula is best represented by large numbers. The oxides of tungsten illustrate this situation. Starting from the idealized material tungsten trioxide, one can generate a series of related materials that are slightly deficient in oxygen. These oxygen-deficient species can be described as WO3-x but in fact they are stoichiometric species with large unit cells with the formulas WnO(3n-2) where n = 20, 24, 25, 40. Thus, the last species can be described with the stoichiometric formula W40O118, whereas the non-stoichiometric description WO2.95 implies a more random distribution of oxide vacancies.Iron(II) sulfide
The monosulfides of the transition metals are often nonstoichiometric. Best known perhaps is iron(II) sulfide (mineral pyrrhotitePyrrhotite
Pyrrhotite is an unusual iron sulfide mineral with a variable iron content: FeS . The FeS endmember is known as troilite. Pyrrhotite is also called magnetic pyrite because the color is similar to pyrite and it is weakly magnetic...
) with a composition Fe(1-x)S (x = 0 to 0.2). The rare stoichiometric FeS endmember
Endmember (mineralogy)
An endmember in mineralogy is a mineral that is at the extreme end of a mineral series in terms of purity. Minerals often can be described as solid solutions with varying compositions of some chemical elements, rather than as substances with an exact chemical formula...
is known as mineral troilite
Pyrrhotite
Pyrrhotite is an unusual iron sulfide mineral with a variable iron content: FeS . The FeS endmember is known as troilite. Pyrrhotite is also called magnetic pyrite because the color is similar to pyrite and it is weakly magnetic...
. Pyrrhotite is remarkable in that it has numerous polytypes, i.e. crystalline forms differing in symmetry (monoclinic or hexagonal
Hexagonal crystal system
In crystallography, the hexagonal crystal system is one of the 7 crystal systems, the hexagonal lattice system is one of the 7 lattice systems, and the hexagonal crystal family is one of the 6 crystal families...
) and composition (Fe7S8, Fe9S10, Fe11S12 and others). These materials are always iron-deficient owing to the presence of lattice defects, namely iron vacancies. Despite those defects, the composition is usually expressed as a ratio of large numbers and the crystals symmetry is relatively high. This means the iron vacancies are not randomly scattered over the crystal, but form certain regular configurations. Those vacancies strongly affect the magnetic properties of pyrrhotite: the magnetism increases with the concentration of vacancies and is absent for the stoichiometric FeS.
Other cases
- Palladium hydridePalladium hydridePalladium hydride is metallic palladium that contains a substantial quantity of hydrogen within its crystal lattice. At room temperature and atmospheric pressure, palladium can absorb up to 900 times its own volume of hydrogen. This process is reversible...
is a nonstoichiometric material of the approximate composition PdHx (0.02 < x < 0.58). This solid conducts hydrogen by virtue of the mobility of the hydrogen atoms within the solid. - The coordination polymer Prussian bluePrussian bluePrussian blue is a dark blue pigment with the idealized formula Fe718. Another name for the color Prussian blue is Berlin blue or, in painting, Parisian blue. Turnbull's blue is the same substance but is made from different reagents....
, nominally Fe7(CN)18 is well known to form non-stoichiometrically. In fact the non-stoichiometric phases exhibit more useful properties associated with the ability of the solid to absorb caesiumCaesiumCaesium or cesium is the chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-gold alkali metal with a melting point of 28 °C , which makes it one of only five elemental metals that are liquid at room temperature...
and thalliumThalliumThallium is a chemical element with the symbol Tl and atomic number 81. This soft gray poor metal resembles tin but discolors when exposed to air. The two chemists William Crookes and Claude-Auguste Lamy discovered thallium independently in 1861 by the newly developed method of flame spectroscopy...
ions.
Oxidation catalysis
Many useful chemicals are produced by the reactions of hydrocarbonHydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons from which one hydrogen atom has been removed are functional groups, called hydrocarbyls....
s with oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
, a conversion that is catalyzed
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
by metal oxides. The process operates via the transfer of "lattice" oxygen to the hydrocarbon substrate, a step that temporarily generates a vacancy. In a subsequent step, the oxygen vacancy is replenished by the O2. Such catalysts rely on the ability of the metal oxide to form phases that are not stoichiometric. An analogous sequence of events describes other kinds of atom-transfer reactions including hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
and hydrodesulfurization
Hydrodesulfurization
Hydrodesulfurization is a catalytic chemical process widely used to remove sulfur from natural gas and from refined petroleum products such as gasoline or petrol, jet fuel, kerosene, diesel fuel, and fuel oils...
catalysed by solid catalysts. These considerations also highlight the fact that stoichiometry is determined by the interior of crystals: the surfaces of crystals often do not follow the stoichiometry of the bulk. The complex structures on surfaces are described by the term "surface reconstruction."

