
Perfect gas
Encyclopedia
In physics
, a perfect gas is a theoretical gas that differs from real gases in a way that makes certain calculations easier to handle. Its behavior is more simplified compared to an ideal gas
(also a theoretical gas). In particular, intermolecular forces are neglected, which means that one can use the ideal gas law without restriction and neglect many complications that may arise from the Van der Waals force
s.
A thermally perfect gas
This type of approximation is useful for modeling, for example, an axial compressor
where temperature fluctuations are usually not large enough to cause any significant deviations from the thermally perfect gas model. Heat capacity is still allowed to vary, though only with temperature, and molecules are not permitted to dissociate. The latter implies temperature limited to 1500 K.
Even more restricted is the calorically perfect gas for which, in addition, the specific heat is assumed to be constant: and
and  .
.
Although this may be the most restrictive model from a temperature perspective, it is accurate enough to make reasonable predictions within the limits specified. A comparison of calculations for one compression stage of an axial compressor
(one with variable Cp, and one with constant Cp) produces a deviation small enough to support this approach. As it turns out, other factors come into play and dominate during this compression cycle. These other effects would have a greater impact on the final calculated result than whether or not Cp was held constant. (examples of these real gas effects include compressor tip-clearance, separation, and boundary layer/frictional losses, etc.)
In these definitions, a perfect gas corresponds to a calorically perfect gas in the other definition, and a semi-perfect gas corresponds to a thermally perfect gas.
Physics
Physics is a natural science that involves the study of matter and its motion through spacetime, along with related concepts such as energy and force. More broadly, it is the general analysis of nature, conducted in order to understand how the universe behaves.Physics is one of the oldest academic...
, a perfect gas is a theoretical gas that differs from real gases in a way that makes certain calculations easier to handle. Its behavior is more simplified compared to an ideal gas
Ideal gas
An ideal gas is a theoretical gas composed of a set of randomly-moving, non-interacting point particles. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics.At normal conditions such as...
(also a theoretical gas). In particular, intermolecular forces are neglected, which means that one can use the ideal gas law without restriction and neglect many complications that may arise from the Van der Waals force
Van der Waals force
In physical chemistry, the van der Waals force , named after Dutch scientist Johannes Diderik van der Waals, is the sum of the attractive or repulsive forces between molecules other than those due to covalent bonds or to the electrostatic interaction of ions with one another or with neutral...
s.
Perfect gas nomenclature
The terms perfect gas and ideal gas are sometimes used interchangeably, depending on the particular field of physics and engineering. Sometimes, other distinctions are made, such as between thermally perfect gas and calorically perfect gas, or between imperfect, semi-perfect, perfect, and ideal gases.Thermally and calorically perfect gas
Along with the definition of a perfect gas, there are also two more simplifications that can be made although various textbooks either omit or combine the following simplifications into a general "perfect gas" definition.A thermally perfect gas
- is in thermodynamic equilibriumThermodynamic equilibriumIn thermodynamics, a thermodynamic system is said to be in thermodynamic equilibrium when it is in thermal equilibrium, mechanical equilibrium, radiative equilibrium, and chemical equilibrium. The word equilibrium means a state of balance...
- is not chemically reacting
- has internal energyInternal energyIn thermodynamics, the internal energy is the total energy contained by a thermodynamic system. It is the energy needed to create the system, but excludes the energy to displace the system's surroundings, any energy associated with a move as a whole, or due to external force fields. Internal...
e, enthalpyEnthalpyEnthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
h, and specific heat Cv that are functions of temperature only and not of pressure, i.e.,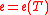 ,
,  ,
,  ,
,  .
.
This type of approximation is useful for modeling, for example, an axial compressor
Axial compressor
Axial compressors are rotating, airfoil-based compressors in which the working fluid principally flows parallel to the axis of rotation. This is in contrast with other rotating compressors such as centrifugal, axi-centrifugal and mixed-flow compressors where the air may enter axially but will have...
where temperature fluctuations are usually not large enough to cause any significant deviations from the thermally perfect gas model. Heat capacity is still allowed to vary, though only with temperature, and molecules are not permitted to dissociate. The latter implies temperature limited to 1500 K.
Even more restricted is the calorically perfect gas for which, in addition, the specific heat is assumed to be constant:
 and
and  .
.Although this may be the most restrictive model from a temperature perspective, it is accurate enough to make reasonable predictions within the limits specified. A comparison of calculations for one compression stage of an axial compressor
Axial compressor
Axial compressors are rotating, airfoil-based compressors in which the working fluid principally flows parallel to the axis of rotation. This is in contrast with other rotating compressors such as centrifugal, axi-centrifugal and mixed-flow compressors where the air may enter axially but will have...
(one with variable Cp, and one with constant Cp) produces a deviation small enough to support this approach. As it turns out, other factors come into play and dominate during this compression cycle. These other effects would have a greater impact on the final calculated result than whether or not Cp was held constant. (examples of these real gas effects include compressor tip-clearance, separation, and boundary layer/frictional losses, etc.)
Perfect, semi-perfect, and imperfect gas
The following distinction can be made, and is, for example, taught in Cambridge University:- For a perfect gas, the ideal-gas law
 holds,
holds, 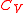 is constant, and
is constant, and  .
. - For a semi-perfect gas, the ideal-gas law holds,
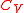 is a function of temperature, and
is a function of temperature, and 
- For an ideal gas, the ideal-gas law holds, and
 holds, without assumptions on temperature and pressure dependence.
holds, without assumptions on temperature and pressure dependence. - For an imperfect gas, the ideal-gas law does not hold,
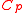 and
and  are functions of temperature and pressure, and
are functions of temperature and pressure, and 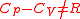 .
.
In these definitions, a perfect gas corresponds to a calorically perfect gas in the other definition, and a semi-perfect gas corresponds to a thermally perfect gas.

