Petasis reaction
Encyclopedia
The Petasis reaction is the chemical reaction
of an amine
, aldehyde
, and vinyl
- or aryl
-boronic acid
to form substituted amines.
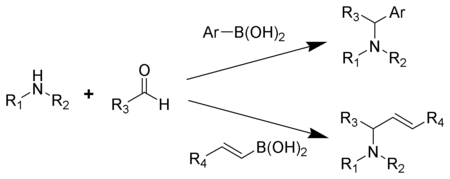 Reported in 1993 by N.A. Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine
Reported in 1993 by N.A. Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine
, the Petasis reaction can be described as a variation of the Mannich reaction
. Rather than generating an enolate to form the substituted amine product, in the Petasis reaction, the vinyl group of the organoboronic acid serves as the nucleophile. In comparison to other methods of generating allyl amines, the Petasis reaction tolerates a multifunctional scaffold, with a variety of amines and organoboronic acids as potential starting materials. Additionally, the reaction does not require anhydrous or inert conditions. As a mild, selective synthesis, the Petasis reaction is useful in generating α-amino acids, and is utilized in combinatorial chemistry
and drug discovery
.
The irreversible C-C bond migration between 3 and 3’ then follows, furnishing desired product 9 with loss of boric acid. All intermediates will ultimately lead to the final product, as the reaction between 3 and 3’ is irreversible, pulling the equilibrium of the entire system towards the final product.
For substrates that have an internal hydroxyl group that can participate in boronic acid coordination, the hydroxyl group in 3 attacks the electrophilic boron, forming a complex that activates the vinyl group as a nucleophile. A bond is formed between the carbon once bound to the boron and the carbon adjacent to the hydroxyl group as the carbon-oxygen bond of the hydroxyl amine breaks. An allyl amine and one equivalent of boronic acid are formed in this process.
syntheses, the Petasis reaction avoids the use of cyanide
and isocyanide
reagents. The amine is mixed with the carbonyl substrate using either dioxane or toluene
as a solvent at 90 oC for 10 minutes. Subsequently, the boronic acid is added to the mixture and product is generated, either after 30 minutes at 90 oC, or after several hours at 25 oC. In α-amino acid synthesis, α-keto acids, such as glyoxylic and pyruvic acid, are stirred in ethanol
, toluene, or dichloromethane
with amines and vinyl boronic acids at 25-50 oC for 12-48 h to give the corresponding β,γ-unsaturated compounds.
One of the most attractive features of the Petasis reaction is its use of boronic acids as a nucleophilic source. Unlike most vinyl substrates, vinyl boronic acids are stable to air and water and can be removed during workup with a simple extraction. Many boronic acid derivatives are easy to prepare and with the advent of the Suzuki coupling, a larger number of them are now commercially available. In the seminal report of the reaction, the organoboronic acids were prepared by hydroboration of terminal alkynes with catecholborane
.
Other methods of generating boronic acids were also reported.
s. Additionally, a variety of substituted amines can be used other than secondary amines. Tertiary aromatic amines, hydrazines, hydroxylamines, sulfonamides, and indoles have all been reported.
Synthesis of allyl amines
The Petasis reaction generates geometrically pure allyl amines. The preparation of the hydroboronic acid reagent gives exclusively cis stereochemistry, consistent with the mechanism of hydroboration. The configuration of the boronic acid is then retained in the olefin. In Petasis’ original article, (E)-vinyl boronic acids gave 100% (E)-allylamine products with yields of 75-96%..
Synthesis of amino acids
The Petasis reaction exhibits high degrees of stereocontrol when a chiral amine or aldehyde is used as a substrate. When certain chiral amines, such as (S)-2-phenylglycinol, are mixed with an α-keto acid and vinyl boronic acid at room temperature, the corresponding allylamine is formed as a single diastereomer. Furthermore, enantiomeric purity can be achieved by hydrogenation of the diastereoselective product. In the reaction with (S)-2-phenylglycinol, (R)-2-phenylglycinol is generated in 76% yield..
Synthesis of Iminodicarboxylic Acid Derivatives
Synthesis of Peptidomimetic Heterocycles
Synthesis of amino alcohols
When a α-hydroxy aldehyde is used as a substrate in the synthesis of β-amino alcohols, a single diastereomer is generated. This reaction forms exclusively anti-product, confirmed by 1H NMR spectroscopy. The product does not undergo racemization, and when enantiomerically pure α-hydroxy aldehydes are used, enantiomeric excess can be achieved. It is believed that the boronic acid first reacted with the chiral hydroxyl group, furnishing a nucleophilic alkenyl boronate, followed by face selective, intramolecular migration of the alkenyl group into the electrophilic iminium carbon, forming the desired C-C bond irreversibly. In the reaction of enantiomerically pure glyceraldehydes, the corresponding 3-amino 1,2-diol product is formed in 70% yield and greater than 99% ee..
Synthesis of Amino Polyols and Amino Sugars
Takemoto and coworkers of Kyoto University recently reported an enantioselective Petasis-type reaction to transform quinolines into respective chiral 1,2-dihydroquinolines (product) using alkenyl boronic acids and chiral thiourea catalyst. Good yields (59-78%) and excellent enantioselectivities (82-96%) are reported.
Takemoto and coworkers observed that addition of chloroformates are required as electrophilic activating agents, and the reaction does not proceed without them. Also, a 1,2-amino alcohol functionality is required on the catalyst for the reaction to proceed stereoselectively. They rationalize these findings by suggesting that the chloroformate reagent reacted with the quinoline nitrogen to make an N-acyated quinolinium intermediate B, which is further activated by electrophilic chiral thiourea. They also suggest that the 1,2-amino alcohol functionality of the catalyst is chelating to the alkenyl boronic acids and that such chelation directed the stereochemical outcome.
With chiral biphenols
Schaus and Lou of Boston University reported the following reaction, in which chiral α-amino acids with various functionalities are conveniently furnished by mixing alkenyl diethyl boronates, secondary amines, glyoxylates and chiral biphenol catalyst in toluene in one-pot:
This reaction tolerates a wide range of functionalities, both on the sides of alkenyl boronates and the secondary amine: the electron-richness of the substrates does not affect the yield and enantioselectivity, and sterically demanding substrates (dialkylsubstituted alkenyl boronates and amines with α-stereocenter) only compromise enantioselectivity slightly. Reaction rates do vary on a case-by-case basis.
Interestingly, under the reported condition, boronic acids substrates failed to give any enantioselectivity. Also, 3Å molecular sieve is used in the reaction system. While the authors did not provide the reason for such usage in the paper, it was speculated that 3Å molecular sieves act as water scavenger and prevent the decomposition of alkenyl diethyl boronates into their respective boronic acids. The catalyst could be recycled from the reaction and reused without compromising yield or enantioselectivity.
In one application the Petasis reaction is used for quick access to a multifunctional scaffold for divergent synthesis
. The reactants are the lactol
derived from L-phenyl-lactic acid
and acetone
, l-phenylalanine methyl ester
and a boronic acid
. The reaction takes place in ethanol at room temperature to give the product, an anti-1,2-amino alcohol with a diastereomeric excess of 99%.
Notice that the authors cannot assess syn-1,2-amino alcohol with this method due to intrinsic mechanistic selectivity, and the authors argue that such intrinsic selectivity hampers their ability to access the full matrix of stereoisomeric products for the usage of small molecule screening. In a recent report, Schaus and co-workers reported that syn amino alcohol can be obtained with the following reaction condition, using a chiral dibromo-biphenol catalyst their group developed:
Although the syn vs. anti diastereomeric ratio ranges from mediocre to good (1.5:1 to 7.5:1), the substrate scope for such reactions remain rather limited, and the diastereoselectivity is found to be dependent on the stereogenic center on the amine starting material.
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
of an amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
, aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
, and vinyl
Vinyl
A vinyl compound is any organic compound that contains a vinyl group ,which are derivatives of ethene, CH2=CH2, with one hydrogen atom replaced with some other group...
- or aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
-boronic acid
Boronic acid
A boronic acid is an alkyl or aryl substituted boric acid containing a carbon–boron bond belonging to the larger class of organoboranes. Boronic acids act as Lewis acids. Their unique feature is that they are capable of forming reversible covalent complexes with sugars, amino acids, hydroxamic...
to form substituted amines.

Naftifine
Naftifine is an allylamine antifungal drug for the topical treatment of tinea pedis, tinea cruris, and tinea corporis ....
, the Petasis reaction can be described as a variation of the Mannich reaction
Mannich reaction
The Mannich reaction is an organic reaction which consists of an amino alkylation of an acidic proton placed next to a carbonyl functional group with formaldehyde and ammonia or any primary or secondary amine. The final product is a β-amino-carbonyl compound also known as a Mannich base...
. Rather than generating an enolate to form the substituted amine product, in the Petasis reaction, the vinyl group of the organoboronic acid serves as the nucleophile. In comparison to other methods of generating allyl amines, the Petasis reaction tolerates a multifunctional scaffold, with a variety of amines and organoboronic acids as potential starting materials. Additionally, the reaction does not require anhydrous or inert conditions. As a mild, selective synthesis, the Petasis reaction is useful in generating α-amino acids, and is utilized in combinatorial chemistry
Combinatorial chemistry
Combinatorial chemistry involves the rapid synthesis or the computer simulation of a large number of different but structurally related molecules or materials...
and drug discovery
Drug discovery
In the fields of medicine, biotechnology and pharmacology, drug discovery is the process by which drugs are discovered or designed.In the past most drugs have been discovered either by identifying the active ingredient from traditional remedies or by serendipitous discovery...
.
Reaction Mechanism
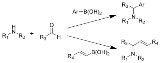
The mechanism of the Petasis reaction is not fully understood; however, it is similar to the Mannich reaction at its early stage. In the Mannich reaction, an imine or iminium salt serves as the electrophile to which the nucleophile adds; however, in the Petasis reaction it is not clear which intermediate serves as the electrophile. Petasis proposes that the reaction is characterized by a complex equilibrium among the three starting materials and various intermediates, and the final product is formed via an irreversible C-C bond forming step. The condensation between amine 1 and carbonyl 2 forms hemiaminal 4, which is in a complex equilibrium with iminium ion 3 and aminal 5. Boronic acid 6 react with hemiaminal 4 and aminal 5 in a reversible fashion via intermediate 7 and 8 respectively, forming again electrophilic iminium ion 3, this time accompanied by nucleophilic boronate 3’. Note that there are no evidences suggesting that boronic acid alone can directly react with iminium ions: In addition to needing acid for an appreciable amount of iminium salt to be generated, it has been shown that vinyl boronic acids do not react efficiently with preformed iminium salts.The irreversible C-C bond migration between 3 and 3’ then follows, furnishing desired product 9 with loss of boric acid. All intermediates will ultimately lead to the final product, as the reaction between 3 and 3’ is irreversible, pulling the equilibrium of the entire system towards the final product.
For substrates that have an internal hydroxyl group that can participate in boronic acid coordination, the hydroxyl group in 3 attacks the electrophilic boron, forming a complex that activates the vinyl group as a nucleophile. A bond is formed between the carbon once bound to the boron and the carbon adjacent to the hydroxyl group as the carbon-oxygen bond of the hydroxyl amine breaks. An allyl amine and one equivalent of boronic acid are formed in this process.
Preparation
The Petasis reaction proceeds under mild conditions, without the use of strong acids, bases, or metals. Unlike the Strecker and UgiUgi
Ugi may refer to:in places:*Ugi Island in the Makira-Ulawa Province of the Solomon Islandsin people:*Camillo Ugi , German footballer*Ivar Karl Ugi , German chemist known for Ugi reactionin other:*Ugi reaction...
syntheses, the Petasis reaction avoids the use of cyanide
Cyanide
A cyanide is a chemical compound that contains the cyano group, -C≡N, which consists of a carbon atom triple-bonded to a nitrogen atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic....
and isocyanide
Isocyanide
An isocyanide is an organic compound with the functional group -N≡C. It is the isomer of the related cyanide , hence the prefix iso....
reagents. The amine is mixed with the carbonyl substrate using either dioxane or toluene
Toluene
Toluene, formerly known as toluol, is a clear, water-insoluble liquid with the typical smell of paint thinners. It is a mono-substituted benzene derivative, i.e., one in which a single hydrogen atom from the benzene molecule has been replaced by a univalent group, in this case CH3.It is an aromatic...
as a solvent at 90 oC for 10 minutes. Subsequently, the boronic acid is added to the mixture and product is generated, either after 30 minutes at 90 oC, or after several hours at 25 oC. In α-amino acid synthesis, α-keto acids, such as glyoxylic and pyruvic acid, are stirred in ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
, toluene, or dichloromethane
Dichloromethane
Dichloromethane is an organic compound with the formula CH2Cl2. This colorless, volatile liquid with a moderately sweet aroma is widely used as a solvent. Although it is not miscible with water, it is miscible with many organic solvents...
with amines and vinyl boronic acids at 25-50 oC for 12-48 h to give the corresponding β,γ-unsaturated compounds.
One of the most attractive features of the Petasis reaction is its use of boronic acids as a nucleophilic source. Unlike most vinyl substrates, vinyl boronic acids are stable to air and water and can be removed during workup with a simple extraction. Many boronic acid derivatives are easy to prepare and with the advent of the Suzuki coupling, a larger number of them are now commercially available. In the seminal report of the reaction, the organoboronic acids were prepared by hydroboration of terminal alkynes with catecholborane
Catecholborane
Catecholborane, or HBcat, is a derivative of catechol and a boron hydride, with the formula C6H4O2BH. It is commonly used in organic syntheses.-Synthesis:...
.
Other methods of generating boronic acids were also reported.
Reaction Scope and Synthetic Applications
A wide variety of functional groups including alcohols, carboxylic acids, and amines are tolerated in the Petasis Reaction. Known substrates that are compatible with reaction conditions include vinylboronate esters, arylboronate esters, and potassium organotrifluoroborateOrganotrifluoroborate
Organotrifluoroborates are organoboron compounds that contain an anion with the general formula [RBF3]−. They can be thought of as protected boronic acids, or as adducts of carbanions and boron trifluoride. Organotrifluoroborates are tolerant of air and moisture and are easy to handle and purify...
s. Additionally, a variety of substituted amines can be used other than secondary amines. Tertiary aromatic amines, hydrazines, hydroxylamines, sulfonamides, and indoles have all been reported.
Synthesis of allyl amines
The Petasis reaction generates geometrically pure allyl amines. The preparation of the hydroboronic acid reagent gives exclusively cis stereochemistry, consistent with the mechanism of hydroboration. The configuration of the boronic acid is then retained in the olefin. In Petasis’ original article, (E)-vinyl boronic acids gave 100% (E)-allylamine products with yields of 75-96%..
Synthesis of amino acids
The Petasis reaction exhibits high degrees of stereocontrol when a chiral amine or aldehyde is used as a substrate. When certain chiral amines, such as (S)-2-phenylglycinol, are mixed with an α-keto acid and vinyl boronic acid at room temperature, the corresponding allylamine is formed as a single diastereomer. Furthermore, enantiomeric purity can be achieved by hydrogenation of the diastereoselective product. In the reaction with (S)-2-phenylglycinol, (R)-2-phenylglycinol is generated in 76% yield..
Synthesis of Iminodicarboxylic Acid Derivatives
Synthesis of Peptidomimetic Heterocycles
Synthesis of amino alcohols
When a α-hydroxy aldehyde is used as a substrate in the synthesis of β-amino alcohols, a single diastereomer is generated. This reaction forms exclusively anti-product, confirmed by 1H NMR spectroscopy. The product does not undergo racemization, and when enantiomerically pure α-hydroxy aldehydes are used, enantiomeric excess can be achieved. It is believed that the boronic acid first reacted with the chiral hydroxyl group, furnishing a nucleophilic alkenyl boronate, followed by face selective, intramolecular migration of the alkenyl group into the electrophilic iminium carbon, forming the desired C-C bond irreversibly. In the reaction of enantiomerically pure glyceraldehydes, the corresponding 3-amino 1,2-diol product is formed in 70% yield and greater than 99% ee..
Synthesis of Amino Polyols and Amino Sugars
Applications in Enantioselective Synthesis
With thiourea catalystTakemoto and coworkers of Kyoto University recently reported an enantioselective Petasis-type reaction to transform quinolines into respective chiral 1,2-dihydroquinolines (product) using alkenyl boronic acids and chiral thiourea catalyst. Good yields (59-78%) and excellent enantioselectivities (82-96%) are reported.
Takemoto and coworkers observed that addition of chloroformates are required as electrophilic activating agents, and the reaction does not proceed without them. Also, a 1,2-amino alcohol functionality is required on the catalyst for the reaction to proceed stereoselectively. They rationalize these findings by suggesting that the chloroformate reagent reacted with the quinoline nitrogen to make an N-acyated quinolinium intermediate B, which is further activated by electrophilic chiral thiourea. They also suggest that the 1,2-amino alcohol functionality of the catalyst is chelating to the alkenyl boronic acids and that such chelation directed the stereochemical outcome.
With chiral biphenols
Schaus and Lou of Boston University reported the following reaction, in which chiral α-amino acids with various functionalities are conveniently furnished by mixing alkenyl diethyl boronates, secondary amines, glyoxylates and chiral biphenol catalyst in toluene in one-pot:
This reaction tolerates a wide range of functionalities, both on the sides of alkenyl boronates and the secondary amine: the electron-richness of the substrates does not affect the yield and enantioselectivity, and sterically demanding substrates (dialkylsubstituted alkenyl boronates and amines with α-stereocenter) only compromise enantioselectivity slightly. Reaction rates do vary on a case-by-case basis.
Interestingly, under the reported condition, boronic acids substrates failed to give any enantioselectivity. Also, 3Å molecular sieve is used in the reaction system. While the authors did not provide the reason for such usage in the paper, it was speculated that 3Å molecular sieves act as water scavenger and prevent the decomposition of alkenyl diethyl boronates into their respective boronic acids. The catalyst could be recycled from the reaction and reused without compromising yield or enantioselectivity.
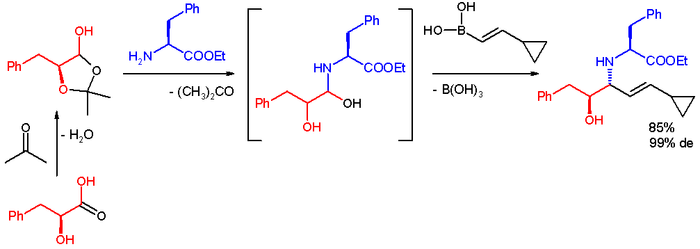
In one application the Petasis reaction is used for quick access to a multifunctional scaffold for divergent synthesis
Divergent synthesis
In chemistry a divergent synthesis is a strategy with the aim to improve the efficiency of chemical synthesis. It is often an alternative to convergent synthesis or linear synthesis....
. The reactants are the lactol
Lactol
In organic chemistry, a lactol is the cyclic equivalent of a hemiacetal or a hemiketal.The compound is formed by the intramolecular nucleophilic addition of a hydroxyl group to the carbonyl group of an aldehyde or a ketone....
derived from L-phenyl-lactic acid
Lactic acid
Lactic acid, also known as milk acid, is a chemical compound that plays a role in various biochemical processes and was first isolated in 1780 by the Swedish chemist Carl Wilhelm Scheele. Lactic acid is a carboxylic acid with the chemical formula C3H6O3...
and acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
, l-phenylalanine methyl ester
Alanine
Alanine is an α-amino acid with the chemical formula CH3CHCOOH. The L-isomer is one of the 20 amino acids encoded by the genetic code. Its codons are GCU, GCC, GCA, and GCG. It is classified as a nonpolar amino acid...
and a boronic acid
Boronic acid
A boronic acid is an alkyl or aryl substituted boric acid containing a carbon–boron bond belonging to the larger class of organoboranes. Boronic acids act as Lewis acids. Their unique feature is that they are capable of forming reversible covalent complexes with sugars, amino acids, hydroxamic...
. The reaction takes place in ethanol at room temperature to give the product, an anti-1,2-amino alcohol with a diastereomeric excess of 99%.

Notice that the authors cannot assess syn-1,2-amino alcohol with this method due to intrinsic mechanistic selectivity, and the authors argue that such intrinsic selectivity hampers their ability to access the full matrix of stereoisomeric products for the usage of small molecule screening. In a recent report, Schaus and co-workers reported that syn amino alcohol can be obtained with the following reaction condition, using a chiral dibromo-biphenol catalyst their group developed:
Although the syn vs. anti diastereomeric ratio ranges from mediocre to good (1.5:1 to 7.5:1), the substrate scope for such reactions remain rather limited, and the diastereoselectivity is found to be dependent on the stereogenic center on the amine starting material.

