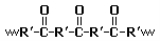
Polyketone
Encyclopedia
| Polyketone | |
|---|---|
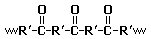 | |
| Density Density The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight... | 1240 kg/m3 |
| Young's modulus Young's modulus Young's modulus is a measure of the stiffness of an elastic material and is a quantity used to characterize materials. It is defined as the ratio of the uniaxial stress over the uniaxial strain in the range of stress in which Hooke's Law holds. In solid mechanics, the slope of the stress-strain... (E) | 1500 MPa Pascal (unit) The pascal is the SI derived unit of pressure, internal pressure, stress, Young's modulus and tensile strength, named after the French mathematician, physicist, inventor, writer, and philosopher Blaise Pascal. It is a measure of force per unit area, defined as one newton per square metre... |
| Tensile strength Tensile strength Ultimate tensile strength , often shortened to tensile strength or ultimate strength, is the maximum stress that a material can withstand while being stretched or pulled before necking, which is when the specimen's cross-section starts to significantly contract... (σt) | 55 MPa Pascal (unit) The pascal is the SI derived unit of pressure, internal pressure, stress, Young's modulus and tensile strength, named after the French mathematician, physicist, inventor, writer, and philosopher Blaise Pascal. It is a measure of force per unit area, defined as one newton per square metre... |
| Elongation @ break | 350 % |
| notch test | 20 kJ/m2 |
| Glass temperature | 15°C |
| melting point Melting point The melting point of a solid is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at standard atmospheric pressure... | 220°C |
| Vicat B | 205 |
| heat transfer coefficient (λ) | 0.27 W/(m·K Kelvin The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all... ) |
| linear expansion coefficient (α) | 11 10−5/K Kelvin The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all... |
| Specific heat (c) | 1.8 kJ/(kg Kilogram The kilogram or kilogramme , also known as the kilo, is the base unit of mass in the International System of Units and is defined as being equal to the mass of the International Prototype Kilogram , which is almost exactly equal to the mass of one liter of water... ·K) |
| Water absorption (ASTM) | 0.5 |
| Price | 3-5 €/kg Kilogram The kilogram or kilogramme , also known as the kilo, is the base unit of mass in the International System of Units and is defined as being equal to the mass of the International Prototype Kilogram , which is almost exactly equal to the mass of one liter of water... |
| # Deformation temperature at 10 kN needle load | |
| source: A.K. vam der Vegt & L.E. Govaert, Polymeren: | |
| van keten tot kunstof, ISBN 90-407-2388-5 | |
Polyketones are a family of high-performance thermoplastic
Thermoplastic
Thermoplastic, also known as a thermosoftening plastic, is a polymer that turns to a liquid when heated and freezes to a very glassy state when cooled sufficiently...
polymers. The polar ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
groups in the polymer backbone of these materials gives rise to a strong attraction between polymer chains, which increases the material's melting point (255 °C for Carilon). Such materials also tend to resist solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
s and have good mechanical properties. Unlike many other engineering plastic
Engineering plastic
Engineering plastics are a group of plastic materials that exhibit superior mechanical and thermal properties in a wide range of conditions over and above more commonly used commodity plastics. The term usually refers to thermoplastic materials rather than thermosetting ones...
s, aliphatic polyketones such as Shell Chemicals
Royal Dutch Shell
Royal Dutch Shell plc , commonly known as Shell, is a global oil and gas company headquartered in The Hague, Netherlands and with its registered office in London, United Kingdom. It is the fifth-largest company in the world according to a composite measure by Forbes magazine and one of the six...
' Carilon (where R' in the diagram is a -CH2CH2- group) are relatively easy to synthesize and can be derived from inexpensive monomer
Monomer
A monomer is an atom or a small molecule that may bind chemically to other monomers to form a polymer; the term "monomeric protein" may also be used to describe one of the proteins making up a multiprotein complex...
s. Carilon is made with a palladium
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
(II) catalyst from ethylene
Ethylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
and carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
. A small fraction of the ethylene is generally replaced with propylene
Propylene
Propene, also known as propylene or methylethylene, is an unsaturated organic compound having the chemical formula C3H6. It has one double bond, and is the second simplest member of the alkene class of hydrocarbons, and it is also second in natural abundance.-Properties:At room temperature and...
to reduce the melting point somewhat. Shell Chemical commercially launched Carilon thermoplastic polymer in the U.S.in 1996, but discontinued it in 2000.
For a discussion of the silicon
Silicon
Silicon is a chemical element with the symbol Si and atomic number 14. A tetravalent metalloid, it is less reactive than its chemical analog carbon, the nonmetal directly above it in the periodic table, but more reactive than germanium, the metalloid directly below it in the table...
containing polymers originally thought to have analogous structures, see silicone
Silicone
Silicones are inert, synthetic compounds with a variety of forms and uses. Typically heat-resistant and rubber-like, they are used in sealants, adhesives, lubricants, medical applications , cookware, and insulation....
polymers.
Industrial production
The ethylene-carbon monoxide co-polymer is most significant. Industrially, this polymer is synthesized either as a methanol slurry, or via a gas phase reaction with immobilized catalysts.Initiation and termination
Where external initiation is not employed for the methanol system, initiation can take place via methanolysis of the palladium(II) precursor, giving either a methoxide or a hydride complex. Termination occurs also by methanolysis. Depending on the end of the growing polymer chain, this results in either an ester or a ketone end group, and regenerating the palladium methoxide or hydride catalysts respectively.Propagation
A mechanism for the propagation of this reaction using a palladium(II)-phenanthrolinePhenanthroline
Phenanthroline is a heterocyclic organic compound. As a bidentate ligand in coordination chemistry, it forms strong complexes with most metal ions...
catalyst has been proposed by Brookhart
Maurice Brookhart
Maurice S. Brookhart is the William R. Kenan, Jr. Professor of Chemistry in the Department of Chemistry at the University of North Carolina....
:
Polyketones are noted for having extremely low defects (double ethylene insertions or double carbonyl insertions, in red):
The activation barrier to give double carbonyl insertions is very high, so it does not occur. Brookhart's mechanistic studies show that the concentration of the alkyl-ethylene palladium complex required to give double ethylene insertions is very low at any one point:
Additionally, the Gibbs energy of activation of the alkyl-ethylene insertion is ~ 3 kcal/mol higher than the corresponding activation barrier for the alkyl-carbon monoxide insertion. As a result, defects occur at an extremely low rate (~ 1 part per million). Tthe industrially-relevant palladium-dppp catalyst has also been investigated.
Importance of bidentate ligands
Where palladium(II) pre-catalysts bearing monodentate phosphine ligands are used in methanol, a relatively high fraction of methyl propionate is produced. In comparison, where chelating diphosphine ligands are used, this side-product is absent. This observation is rationalized: the bis(phosphine) complex can undergo cis-trans isomerization to give the sterically favored trans isomer. The propionyl ligand is now trans- to the open coordination site or ethylene ligand, and is unable to undergo migratory insertionMigratory insertion
A migratory insertion is a type of reaction in organometallic chemistry wherein two ligands on a metal complex combine. It is a subset of reactions that very closely resembles the insertion reactions, and both are differentiated by the mechanism that leads to the resulting stereochemistry of the...
. Instead, solvolysis by methanol occurs, which gives the undesired methyl propionate side-product.

