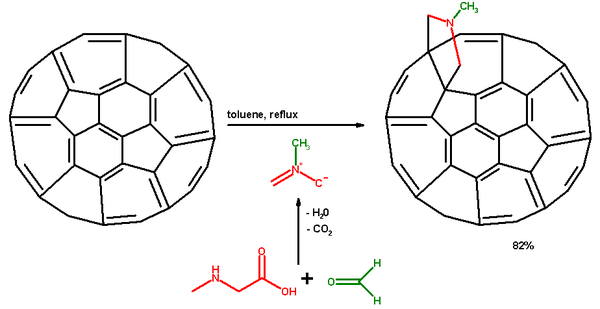
Prato reaction
Encyclopedia
The Prato reaction in fullerene chemistry
describes the functionalization of fullerene
s and nanotube
s with azomethine ylides in a 1,3-dipolar cycloaddition
. The amino acid sarcosine
reacts with paraformaldehyde
when heated at reflux
in toluene
to an ylide
which reacts with a double in a 6,6 ring position in a fullerene
in a 1,3-dipolar cycloaddition
to yield a N-methylpyrrolidine
derivative or pyrrolidinofullerene or pyrrolidino3,4:1,2 [60]fullerene in 82% yield.
chain the resulting nanotubes are soluble in common solvents such chloroform
and acetone
. Another characteristic of the treated nanotubes is their larger aggregate dimensions compared to untreated nanotubes.
In an alternative method a nanotube addition is performed with the N-oxide of trimethylamine
and LDA
at reflux in tetrahydrofuran
with an efficiency of 1 functional group in 16 nanotube carbon atoms. When the amine also carries an aromatic group such as pyrene
the reaction takes place even at room temperature
because this group preorganizes itself to the nanotube surface prior to reaction by pi stacking.
or Diels-Alder reaction
s this reaction can be reversed. A thermal retro-cycloaddition of a pyrrolidinofullerene with a strong dipolarophile such as maleic acid
and a catalyst such as Wilkinson's catalyst
or copper triflate in 1,2-dichlorobenzene
at reflux
8 to 18 hours regenerates the pristine C60 fullerene . The dipolarophile is required in a 30 fold excess and traps the ylide
driving the reaction to completion. The N-methylpyrrolidine
derivative reacts poorly (5% yield) and for a successful reaction the nitrogen ring also requires substitution in the α-position with methyl, phenyl or carboxylic ester groups.
Other methods have been investigated: by applying heat or via a combination of ionic liquid
and microwave chemistry
.
Fullerene chemistry
Fullerene chemistry is a field of organic chemistry devoted to the chemical properties of fullerenes. Research in this field is driven by the need to functionalize fullerenes and tune their properties. For example fullerene is notoriously insoluble and adding a suitable group can enhance...
describes the functionalization of fullerene
Fullerene
A fullerene is any molecule composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Spherical fullerenes are also called buckyballs, and they resemble the balls used in association football. Cylindrical ones are called carbon nanotubes or buckytubes...
s and nanotube
Carbon nanotube
Carbon nanotubes are allotropes of carbon with a cylindrical nanostructure. Nanotubes have been constructed with length-to-diameter ratio of up to 132,000,000:1, significantly larger than for any other material...
s with azomethine ylides in a 1,3-dipolar cycloaddition
1,3-dipolar cycloaddition
The 1,3-dipolar cycloaddition, also known as the Huisgen cycloaddition or Huisgen reaction, is an organic chemical reaction belonging to the larger class of concerted, pericyclic cycloadditions. It is the reaction between a 1,3-dipole and a dipolarophile, most of which are substituted alkenes, to...
. The amino acid sarcosine
Sarcosine
Sarcosine, also known as N-methylglycine, is an intermediate and byproduct in glycine synthesis and degradation. Sarcosine is metabolized to glycine by the enzyme sarcosine dehydrogenase, while glycine-N-methyl transferase generates sarcosine from glycine. Sarcosine is a natural amino acid found in...
reacts with paraformaldehyde
Paraformaldehyde
Paraformaldehyde is the smallest polyoxymethylene, it is the condensation reaction product of formaldehyde with a typical degree of polymerization of 8–100 units. Paraformaldehyde commonly has a slight odor of formaldehyde due to decomposition...
when heated at reflux
Reflux
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations...
in toluene
Toluene
Toluene, formerly known as toluol, is a clear, water-insoluble liquid with the typical smell of paint thinners. It is a mono-substituted benzene derivative, i.e., one in which a single hydrogen atom from the benzene molecule has been replaced by a univalent group, in this case CH3.It is an aromatic...
to an ylide
Ylide
An ylide or ylid is a neutral dipolar molecule containing a formally negatively charged atom directly attached to a hetero atom with a formal positive charge , and in which both atoms have full octets of electrons. Ylides are thus 1,2-dipolar compounds...
which reacts with a double in a 6,6 ring position in a fullerene
Fullerene
A fullerene is any molecule composed entirely of carbon, in the form of a hollow sphere, ellipsoid, or tube. Spherical fullerenes are also called buckyballs, and they resemble the balls used in association football. Cylindrical ones are called carbon nanotubes or buckytubes...
in a 1,3-dipolar cycloaddition
1,3-dipolar cycloaddition
The 1,3-dipolar cycloaddition, also known as the Huisgen cycloaddition or Huisgen reaction, is an organic chemical reaction belonging to the larger class of concerted, pericyclic cycloadditions. It is the reaction between a 1,3-dipole and a dipolarophile, most of which are substituted alkenes, to...
to yield a N-methylpyrrolidine
Pyrrolidine
Pyrrolidine, also known as tetrahydropyrrole, is an organic compound with the molecular formula C4H9N. It is a cyclic secondary amine with a five-membered heterocycle containing four carbon atoms and one nitrogen atom...
derivative or pyrrolidinofullerene or pyrrolidino

Applications
In one application a liquid fullerene is obtained when the pyrrolidone substituent is a 2,4,6-tris(alkyloxy)phenyl group although a small amount of solvent is still required.Nanotubes
This method is also used in the functionalization of single wall nanotubes . When the amino acid is modified with a glycineGlycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
chain the resulting nanotubes are soluble in common solvents such chloroform
Chloroform
Chloroform is an organic compound with formula CHCl3. It is one of the four chloromethanes. The colorless, sweet-smelling, dense liquid is a trihalomethane, and is considered somewhat hazardous...
and acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
. Another characteristic of the treated nanotubes is their larger aggregate dimensions compared to untreated nanotubes.
In an alternative method a nanotube addition is performed with the N-oxide of trimethylamine
Trimethylamine
Trimethylamine is an organic compound with the formula N3. This colorless, hygroscopic, and flammable tertiary amine has a strong "fishy" odor in low concentrations and an ammonia-like odor at higher concentrations...
and LDA
Lithium diisopropylamide
Lithium diisopropylamide is the chemical compound with the formula [2CH]2NLi. Generally abbreviated LDA, it is a strong base used in organic chemistry for the deprotonation of weakly acidic compounds. The reagent has been widely accepted because it is soluble in non-polar organic solvents and it...
at reflux in tetrahydrofuran
Tetrahydrofuran
Tetrahydrofuran is a colorless, water-miscible organic liquid with low viscosity at standard temperature and pressure. This heterocyclic compound has the chemical formula 4O. As one of the most polar ethers with a wide liquid range, it is a useful solvent. Its main use, however, is as a precursor...
with an efficiency of 1 functional group in 16 nanotube carbon atoms. When the amine also carries an aromatic group such as pyrene
Pyrene
Pyrene is a polycyclic aromatic hydrocarbon consisting of four fused benzene rings, resulting in a flat aromatic system. The chemical formula is . This colourless solid is the smallest peri-fused PAH...
the reaction takes place even at room temperature
Room temperature
-Comfort levels:The American Society of Heating, Refrigerating and Air-Conditioning Engineers has listings for suggested temperatures and air flow rates in different types of buildings and different environmental circumstances. For example, a single office in a building has an occupancy ratio per...
because this group preorganizes itself to the nanotube surface prior to reaction by pi stacking.
Retro-Prato reaction
Just as in other fullerene reactions like the Bingel reactionBingel reaction
The Bingel reaction in fullerene chemistry is a fullerene cyclopropanation reaction to a methanofullerene first discovered by C. Bingel in 1993 with the bromo derivative of diethyl malonate in the presence of a base such as sodium hydride or DBU...
or Diels-Alder reaction
Diels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
s this reaction can be reversed. A thermal retro-cycloaddition of a pyrrolidinofullerene with a strong dipolarophile such as maleic acid
Maleic acid
Maleic acid is an organic compound that is a dicarboxylic acid, a molecule with two carboxyl groups. Maleic acid is the cis-isomer of butenedioic acid, whereas fumaric acid is the trans-isomer...
and a catalyst such as Wilkinson's catalyst
Wilkinson's catalyst
Wilkinson's catalyst is the common name for chlorotrisrhodium, a coordination compound with the formula RhCl3 . It is named after the late organometallic chemist and 1973 Nobel Laureate, Sir Geoffrey Wilkinson who popularized its use.-Structure and basic properties:The compound is a square planar,...
or copper triflate in 1,2-dichlorobenzene
1,2-Dichlorobenzene
1,2-Dichlorobenzene, or orthodichlorobenzene , is an organic compound with the formula C6H4Cl2. This colourless liquid is poorly soluble in water but miscible with most organic solvents...
at reflux
Reflux
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations...
8 to 18 hours regenerates the pristine C60 fullerene . The dipolarophile is required in a 30 fold excess and traps the ylide
Ylide
An ylide or ylid is a neutral dipolar molecule containing a formally negatively charged atom directly attached to a hetero atom with a formal positive charge , and in which both atoms have full octets of electrons. Ylides are thus 1,2-dipolar compounds...
driving the reaction to completion. The N-methylpyrrolidine
Pyrrolidine
Pyrrolidine, also known as tetrahydropyrrole, is an organic compound with the molecular formula C4H9N. It is a cyclic secondary amine with a five-membered heterocycle containing four carbon atoms and one nitrogen atom...
derivative reacts poorly (5% yield) and for a successful reaction the nitrogen ring also requires substitution in the α-position with methyl, phenyl or carboxylic ester groups.
Other methods have been investigated: by applying heat or via a combination of ionic liquid
Ionic liquid
An ionic liquid is a salt in the liquid state. In some contexts, the term has been restricted to salts whose melting point is below some arbitrary temperature, such as . While ordinary liquids such as water and gasoline are predominantly made of electrically neutral molecules, ILs are largely made...
and microwave chemistry
Microwave chemistry
Microwave chemistry is the science of applying microwave irradiation to chemical reactions. Microwaves act as high frequency electric fields and will generally heat any material containing mobile electric charges, such as polar molecules in a solvent or conducting ions in a solid...
.

