
Pyrosulfate
Encyclopedia
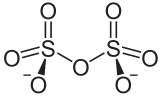
It has a dichromate like structure and can be visualised as two corner sharing SO4 tetrahedra, with a bridging oxygen atom.
In this compound sulfur has an oxidation state
Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
of +6. Disulfate is the conjugate base of the hydrogen disulfate (hydrogen pyrosulfate) ion HS2O7-, which in turn is the conjungate base of disulfuric acid
Disulfuric acid
Disulfuric acid is an oxoacid of sulfur. It is a major constituent of fuming sulfuric acid, oleum, and this is how most chemists encounter it. It is also a minor constituent of liquid anhydrous sulfuric acid due to the equilibria:...
(pyrosulfuric acid).

