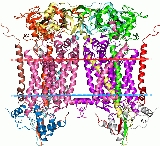
Rieske protein
Encyclopedia
Rieske proteins are iron-sulfur protein
(ISP) components of cytochrome bc1 complexes and cytochrome b6f complexes
which were first discovered and isolated by John S. Rieske and co-workers in 1964. It is a unique [2Fe-2S] cluster in that one of the two Fe atoms is coordinated by two histidine residues rather than two cysteine residues. They have since been found in plants, animals, and bacteria with widely ranging electron reduction potentials from -150 to +400 mV.
and cytochrome c
, generating an electrochemical potential, which is linked to ATP synthesis.
The complex consists of three subunits in most bacteria, and nine in mitochondria: both bacterial and mitochondrial complexes contain cytochrome b and cytochrome c1 subunits, and an iron-sulphur 'Rieske' subunit, which contains a high potential 2Fe-2S cluster. The mitochondrial form also includes six other subunits that do not possess redox centres. Plastoquinone-plastocyanin reductase (b6f complex), present in cyanobacteria and the chloroplasts of plants, catalyses the oxidoreduction of plastoquinol and cytochrome f. This complex, which is functionally similar to ubiquinol-cytochrome c reductase, comprises cytochrome b6, cytochrome f and Rieske subunits.
The Rieske subunit acts by binding either a ubiquinol
or plastoquinol anion, transferring an electron to the 2Fe-2S cluster, then releasing the electron to the cytochrome c
or cytochrome f
haem iron. The reduction of the Rieske center increases the affinity of the subunit by several orders of magnitude, stabilizing the semiquinone radical at the Q(P) site. The Rieske domain has a [2Fe-2S] centre. Two conserved cysteines coordinate one Fe ion while the other Fe ion is coordinated by two conserved histidines. The 2Fe-2S cluster is bound in the highly conserved C-terminal region of the Rieske subunit.
, aromatic-ring-hydroxylating dioxygenases
(phthalate dioxygenase, benzene
, naphthalene
and toluene 1,2-dioxygenases) and arsenite oxidase (EC
1.20.98.1). Comparison of amino acid sequences has revealed the following consensus sequence:
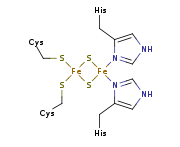
residues and the other is coordinated by two histidine
residues through the Nδ atoms. The ligands coordinating the cluster originate from two loops; each loop contributes one Cys and one His.
Naphthalene 1,2-dioxygenase Rieske ferredoxin - InterPro entry for Rieske [2Fe-2S] region
Iron-sulfur protein
Iron-sulfur proteins are proteins characterized by the presence of iron-sulfur clusters containing sulfide-linked di-, tri-, and tetrairon centers in variable oxidation states...
(ISP) components of cytochrome bc1 complexes and cytochrome b6f complexes
Cytochrome b6f complex
The cytochrome b6f complex is an enzyme found in the thylakoid membrane in chloroplasts of plants, cyanobacteria, and green algae, catalyzing the transfer of electrons from plastoquinol to plastocyanin...
which were first discovered and isolated by John S. Rieske and co-workers in 1964. It is a unique [2Fe-2S] cluster in that one of the two Fe atoms is coordinated by two histidine residues rather than two cysteine residues. They have since been found in plants, animals, and bacteria with widely ranging electron reduction potentials from -150 to +400 mV.
Biological function (in oxidative phosphorylation systems)
Ubiquinol-cytochrome-c reductase (also known as bc1 complex or complex III) is an enzyme complex of bacterial and mitochondrial oxidative phosphorylation systems. It catalyses the oxidoreduction of the mobile redox components ubiquinolUbiquinol
Ubiquinol is an electron-rich form of coenzyme Q10.The natural ubiquinol form of coenzyme Q10 is 2,3-dimethoxy-5-methyl-6-poly prenyl-1,4-benzoquinol, where the polyprenylated side chain is 9-10 units long in mammals...
and cytochrome c
Cytochrome c
The Cytochrome complex, or cyt c is a small heme protein found loosely associated with the inner membrane of the mitochondrion. It belongs to the cytochrome c family of proteins. Cytochrome c is a highly soluble protein, unlike other cytochromes, with a solubility of about 100 g/L and is an...
, generating an electrochemical potential, which is linked to ATP synthesis.
The complex consists of three subunits in most bacteria, and nine in mitochondria: both bacterial and mitochondrial complexes contain cytochrome b and cytochrome c1 subunits, and an iron-sulphur 'Rieske' subunit, which contains a high potential 2Fe-2S cluster. The mitochondrial form also includes six other subunits that do not possess redox centres. Plastoquinone-plastocyanin reductase (b6f complex), present in cyanobacteria and the chloroplasts of plants, catalyses the oxidoreduction of plastoquinol and cytochrome f. This complex, which is functionally similar to ubiquinol-cytochrome c reductase, comprises cytochrome b6, cytochrome f and Rieske subunits.
The Rieske subunit acts by binding either a ubiquinol
Ubiquinol
Ubiquinol is an electron-rich form of coenzyme Q10.The natural ubiquinol form of coenzyme Q10 is 2,3-dimethoxy-5-methyl-6-poly prenyl-1,4-benzoquinol, where the polyprenylated side chain is 9-10 units long in mammals...
or plastoquinol anion, transferring an electron to the 2Fe-2S cluster, then releasing the electron to the cytochrome c
Cytochrome c
The Cytochrome complex, or cyt c is a small heme protein found loosely associated with the inner membrane of the mitochondrion. It belongs to the cytochrome c family of proteins. Cytochrome c is a highly soluble protein, unlike other cytochromes, with a solubility of about 100 g/L and is an...
or cytochrome f
Cytochrome f
Cytochrome f is the largest subunit of cytochrome b6f complex . In its structure and functions, the cytochrome b6f complex bears extensive analogy to the cytochrome bc1 complex of mitochondria and photosynthetic purple bacteria...
haem iron. The reduction of the Rieske center increases the affinity of the subunit by several orders of magnitude, stabilizing the semiquinone radical at the Q(P) site. The Rieske domain has a [2Fe-2S] centre. Two conserved cysteines coordinate one Fe ion while the other Fe ion is coordinated by two conserved histidines. The 2Fe-2S cluster is bound in the highly conserved C-terminal region of the Rieske subunit.
Rieske protein family
The homologues of the Rieske proteins include ISP components of cytochrome b6f complexCytochrome b6f complex
The cytochrome b6f complex is an enzyme found in the thylakoid membrane in chloroplasts of plants, cyanobacteria, and green algae, catalyzing the transfer of electrons from plastoquinol to plastocyanin...
, aromatic-ring-hydroxylating dioxygenases
Aromatic-ring-hydroxylating dioxygenases
Aromatic-ring-hydroxylating dioxygenases incorporate two atoms of dioxygen into their substrates in the dihydroxylation reaction. The product is cis-1,2-dihydroxycyclohexadiene, which is subsequently converted to benzene glycol by a cis-diol dehydrogenase.A large family of multicomponent...
(phthalate dioxygenase, benzene
Benzene
Benzene is an organic chemical compound. It is composed of 6 carbon atoms in a ring, with 1 hydrogen atom attached to each carbon atom, with the molecular formula C6H6....
, naphthalene
Naphthalene
Naphthalene is an organic compound with formula . It is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 ppm by mass. As an aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings...
and toluene 1,2-dioxygenases) and arsenite oxidase (EC
EC number
The Enzyme Commission number is a numerical classification scheme for enzymes, based on the chemical reactions they catalyze....
1.20.98.1). Comparison of amino acid sequences has revealed the following consensus sequence:
- Cys-Xaa-His-(Xaa)15–17-Cys-Xaa-Xaa-His

3D structure
The crystal structures of a number of Rieske proteins are known. The overall fold, comprising two subdomains, is dominated by antiparallel β-structure and contains variable numbers of α-helices. The smaller "cluster-binding" subdomains in mitochondrial and chloroplast proteins are virtually identical, whereas the large subdomains are substantially different in spite of a common folding topology. The [Fe2S2] cluster-binding subdomains have the topology of an incomplete antiparallel β-barrel. One iron atom of the Rieske [Fe2S2] cluster in the domain is coordinated by two cysteineCysteine
Cysteine is an α-amino acid with the chemical formula HO2CCHCH2SH. It is a non-essential amino acid, which means that it is biosynthesized in humans. Its codons are UGU and UGC. The side chain on cysteine is thiol, which is polar and thus cysteine is usually classified as a hydrophilic amino acid...
residues and the other is coordinated by two histidine
Histidine
Histidine Histidine, an essential amino acid, has a positively charged imidazole functional group. It is one of the 22 proteinogenic amino acids. Its codons are CAU and CAC. Histidine was first isolated by German physician Albrecht Kossel in 1896. Histidine is an essential amino acid in humans...
residues through the Nδ atoms. The ligands coordinating the cluster originate from two loops; each loop contributes one Cys and one His.
Subfamilies
- Rieske iron-sulphur protein, C-terminal
- Arsenite oxidase, small subunit
External links
- X-ray structure of Rieske protein (water-soluble fragment) of the bovine mitochondrial cytochrome bc1 complex - X-ray structure of Rieske protein (water-soluble fragment) of the spinach chloroplast cytochrome b6 fcomplex - X-ray structure of Rieske-type ferredoxin associated with biphenyl dioxygenase from Burkholderia cepacia - X-ray structure of Rieske subunit of arsenite oxidase from Alcaligenes faecalis - X-ray structure of the Sphingomonas yanoikuyae B1 Rieske ferredoxin - X-ray structure of the PseudomonasPseudomonas
Pseudomonas is a genus of gammaproteobacteria, belonging to the family Pseudomonadaceae containing 191 validly described species.Recently, 16S rRNA sequence analysis has redefined the taxonomy of many bacterial species. As a result, the genus Pseudomonas includes strains formerly classified in the...
Naphthalene 1,2-dioxygenase Rieske ferredoxin - InterPro entry for Rieske [2Fe-2S] region

