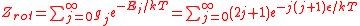Rotational partition function
Encyclopedia
Rotational energies
are quantized. For a diatomic molecule like CO or HCl, the allowed rotational energies are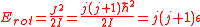
The number of degenerate states
g_j for each level j is 2j+1. The rotational partition function is therefore
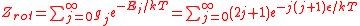
Rotational energy
The rotational energy or angular kinetic energy is the kinetic energy due to the rotation of an object and is part of its total kinetic energy...
are quantized. For a diatomic molecule like CO or HCl, the allowed rotational energies are
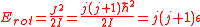
The number of degenerate states
Degenerate energy level
In physics, two or more different quantum states are said to be degenerate if they are all at the same energy level. Statistically this means that they are all equally probable of being filled, and in Quantum Mechanics it is represented mathematically by the Hamiltonian for the system having more...
g_j for each level j is 2j+1. The rotational partition function is therefore