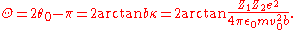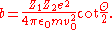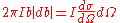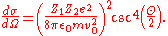Rutherford scattering
Encyclopedia
In physics
, Rutherford scattering is a phenomenon that was explained by Ernest Rutherford
in 1911, and led to the development of the Rutherford model
(planetary model) of the atom
, and eventually to the Bohr model
. It is now exploited by the materials analytical technique
Rutherford backscattering. Rutherford scattering is also sometimes referred to as Coulomb scattering because it relies only upon static electric
(Coulomb) force
s, and the minimal distance between particles is set only by this potential. The classical Rutherford scattering of alpha particles against gold nuclei is an example of "elastic scattering" because the energy and velocity of the outgoing scattered particle is the same as that with which it began.
Rutherford also later analyzed inelastic scattering
when he projected alpha particles against hydrogen nuclei (protons), and this latter process is not classical Rutherford scattering, although it was first observed by him. At the end of such processes, non-coulombic forces come into play. These forces, and also energy gained from the scattering particle by the lighter target, change the scattering results in fundamental ways which suggest structural information about the target. A similar process probed the insides of nuclei in the 1960s, and is called deep inelastic scattering
.
The initial discovery was made by Hans Geiger and Ernest Marsden
in 1909 when they performed the gold foil experiment under the direction of Rutherford, in which they fired a beam of alpha particle
s (helium
nuclei) at layers of gold
leaf only a few atoms thick. At the time of the experiment, the atom was thought to be analogous to a plum pudding (as proposed by J.J. Thomson), with the negative charges (the plums) found throughout a positive sphere (the pudding). If the plum-pudding model were correct, the positive “pudding”, being more spread out than in the current model of a concentrated nucleus
, would not be able to exert such large coulombic forces, and the alpha particles should only be deflected by small angles as they pass through.
However, the intriguing results showed that around 1 in 8000 alpha particles were deflected by very large angles (over 90°), while the rest passed straight through with little or no deflection. From this, Rutherford concluded that the majority of the mass
was concentrated in a minute, positively charged region (the nucleus) surrounded by electrons. When a (positive) alpha particle approached sufficiently close to the nucleus, it was repelled strongly enough to rebound at high angles. The small size of the nucleus explained the small number of alpha particles that were repelled in this way. Rutherford showed, using the method below, that the size of the nucleus was less than about 10−14 m (how much less than this size, Rutherford could not tell from this experiment alone; see more below on this problem of lowest possible size).
interacting under a central force can be decoupled into the motion of the center of mass and the motion of the particles relative to one another. For the case of light alpha particles scattering off heavy nuclei, as in the experiment performed by Rutherford, the reduced mass is essentially the mass of the alpha particle and the nucleus off of which it scatters is essentially stationary in the lab frame.
Substituting into the Binet equation
yields the equation of trajectory
where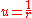 ,
, 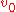 is the speed at infinity, and
is the speed at infinity, and  is the impact parameter
is the impact parameter
.
The general solution of the above differential equation is
and the boundary condition is
Then we can find that
The deflection angle Θ can be seen from the graph or solving as
as
b can be solved readily
To find the scattering cross section from this result consider its definition
Since the scattering angle is uniquely determined for a given and
and  , the number of particles scattered into an angle between
, the number of particles scattered into an angle between  and
and  must be the same as the number of particles with associated impact parameters between
must be the same as the number of particles with associated impact parameters between  and
and  . For an incident intensity
. For an incident intensity 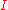 , this implies the following equality
, this implies the following equality
For a radially symmetric scattering potential, as in the case of the Coulombic potential, , yielding the expression for the scattering cross section
, yielding the expression for the scattering cross section
Finally, plugging in the previously derived expression for the impact parameter we find the Rutherford scattering cross section
we find the Rutherford scattering cross section
of the alpha particle is turned into potential energy
and the particle is at rest. The distance from the centre of the alpha particle to the centre of the nucleus (b) at this point is a maximum value for the radius, if it is evident from the experiment that the particles have not hit the nucleus.
Applying the inverse-square law
between the charges on the electron and nucleus, one can write:

Rearranging:
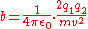
For an alpha particle:
Substituting these in gives the value of about 2.7×10−14 m. (The true radius is about 7.3×10−15 m.) The true radius of the nucleus is not recovered in these experiments because the alphas do not have enough energy to penetrate to more than 27 fm of the nuclear center, as noted, when the actual radius of gold is 7.3 fm. Rutherford realized this, and also realized that actual impact of the alphas on gold causing any force-deviation from that of the 1/r coulomb potential would change the form of his scattering curve at high scattering angles (the smallest impact parameter
s) from a hyperbola
to something else. This was not seen, indicating that the gold had not been "hit" so that Rutherford only knew the gold nucleus (or the sum of the gold and alpha radii) was smaller than 27 fm (2.7×10−14 m)
in Rutherford backscattering spectroscopy (RBS) to detect heavy
elements in a lower atomic number matrix, like for example heavy
metal impurities in semiconductors. In fact, the technique was also
the first local analytical technique applied on the moon, as an alpha-scattering
surface analysis instrument was operating just before
the Surveyor 4
mission
impacted the lunar surface. The same type of instrument was
landed on the lunar surface for more leisurely period of data acquisition
on Surveyors 5 through 7.
), is called Mott scattering
.
Physics
Physics is a natural science that involves the study of matter and its motion through spacetime, along with related concepts such as energy and force. More broadly, it is the general analysis of nature, conducted in order to understand how the universe behaves.Physics is one of the oldest academic...
, Rutherford scattering is a phenomenon that was explained by Ernest Rutherford
Ernest Rutherford
Ernest Rutherford, 1st Baron Rutherford of Nelson OM, FRS was a New Zealand-born British chemist and physicist who became known as the father of nuclear physics...
in 1911, and led to the development of the Rutherford model
Rutherford model
The Rutherford model or planetary model is a model of the atom devised by Ernest Rutherford. Rutherford directed the famous Geiger-Marsden experiment in 1909, which suggested on Rutherford's 1911 analysis that the so-called "plum pudding model" of J. J. Thomson of the atom was incorrect...
(planetary model) of the atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
, and eventually to the Bohr model
Bohr model
In atomic physics, the Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with electrostatic forces providing attraction,...
. It is now exploited by the materials analytical technique
Analytical technique
An analytical technique is a method that is used to determine the concentration of a chemical compound or chemical element. There are a wide variety of techniques used for analysis, from simple weighing to titrations to very advanced techniques using highly specialized instrumentation...
Rutherford backscattering. Rutherford scattering is also sometimes referred to as Coulomb scattering because it relies only upon static electric
Static electricity
Static electricity refers to the build-up of electric charge on the surface of objects. The static charges remain on an object until they either bleed off to ground or are quickly neutralized by a discharge. Static electricity can be contrasted with current electricity, which can be delivered...
(Coulomb) force
Force
In physics, a force is any influence that causes an object to undergo a change in speed, a change in direction, or a change in shape. In other words, a force is that which can cause an object with mass to change its velocity , i.e., to accelerate, or which can cause a flexible object to deform...
s, and the minimal distance between particles is set only by this potential. The classical Rutherford scattering of alpha particles against gold nuclei is an example of "elastic scattering" because the energy and velocity of the outgoing scattered particle is the same as that with which it began.
Rutherford also later analyzed inelastic scattering
Inelastic scattering
In particle physics and chemistry, inelastic scattering is a fundamental scattering process in which the kinetic energy of an incident particle is not conserved . In an inelastic scattering process, some of the energy of the incident particle is lost or gained...
when he projected alpha particles against hydrogen nuclei (protons), and this latter process is not classical Rutherford scattering, although it was first observed by him. At the end of such processes, non-coulombic forces come into play. These forces, and also energy gained from the scattering particle by the lighter target, change the scattering results in fundamental ways which suggest structural information about the target. A similar process probed the insides of nuclei in the 1960s, and is called deep inelastic scattering
Deep Inelastic Scattering
Deep inelastic scattering is the name given to a process used to probe the insides of hadrons , using electrons, muons and neutrinos. It provided the first convincing evidence of the reality of quarks, which up until that point had been considered by many to be a purely mathematical phenomenon...
.
The initial discovery was made by Hans Geiger and Ernest Marsden
Ernest Marsden
Sir Ernest Marsden was an English-New Zealand physicist. He was born in East Lancashire, living in Rishton and educated at Queen Elizabeth's Grammar School, Blackburn, where an inter-house trophy rewarding academic excellence bears his name.He met Ernest Rutherford at the University of Manchester...
in 1909 when they performed the gold foil experiment under the direction of Rutherford, in which they fired a beam of alpha particle
Alpha particle
Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus, which is classically produced in the process of alpha decay, but may be produced also in other ways and given the same name...
s (helium
Helium
Helium is the chemical element with atomic number 2 and an atomic weight of 4.002602, which is represented by the symbol He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas group in the periodic table...
nuclei) at layers of gold
Gold
Gold is a chemical element with the symbol Au and an atomic number of 79. Gold is a dense, soft, shiny, malleable and ductile metal. Pure gold has a bright yellow color and luster traditionally considered attractive, which it maintains without oxidizing in air or water. Chemically, gold is a...
leaf only a few atoms thick. At the time of the experiment, the atom was thought to be analogous to a plum pudding (as proposed by J.J. Thomson), with the negative charges (the plums) found throughout a positive sphere (the pudding). If the plum-pudding model were correct, the positive “pudding”, being more spread out than in the current model of a concentrated nucleus
Atomic nucleus
The nucleus is the very dense region consisting of protons and neutrons at the center of an atom. It was discovered in 1911, as a result of Ernest Rutherford's interpretation of the famous 1909 Rutherford experiment performed by Hans Geiger and Ernest Marsden, under the direction of Rutherford. The...
, would not be able to exert such large coulombic forces, and the alpha particles should only be deflected by small angles as they pass through.
However, the intriguing results showed that around 1 in 8000 alpha particles were deflected by very large angles (over 90°), while the rest passed straight through with little or no deflection. From this, Rutherford concluded that the majority of the mass
Mass
Mass can be defined as a quantitive measure of the resistance an object has to change in its velocity.In physics, mass commonly refers to any of the following three properties of matter, which have been shown experimentally to be equivalent:...
was concentrated in a minute, positively charged region (the nucleus) surrounded by electrons. When a (positive) alpha particle approached sufficiently close to the nucleus, it was repelled strongly enough to rebound at high angles. The small size of the nucleus explained the small number of alpha particles that were repelled in this way. Rutherford showed, using the method below, that the size of the nucleus was less than about 10−14 m (how much less than this size, Rutherford could not tell from this experiment alone; see more below on this problem of lowest possible size).
Derivation
The differential cross section can be derived from the equations of motion for a particle interacting with a central potential. In general, the equations of motion describing two particlesTwo-body problem
In classical mechanics, the two-body problem is to determine the motion of two point particles that interact only with each other. Common examples include a satellite orbiting a planet, a planet orbiting a star, two stars orbiting each other , and a classical electron orbiting an atomic nucleus In...
interacting under a central force can be decoupled into the motion of the center of mass and the motion of the particles relative to one another. For the case of light alpha particles scattering off heavy nuclei, as in the experiment performed by Rutherford, the reduced mass is essentially the mass of the alpha particle and the nucleus off of which it scatters is essentially stationary in the lab frame.
Substituting into the Binet equation
Binet equation
The Binet equation, derived by Jacques Philippe Marie Binet, provides the form of a central force given the shape of the orbital motion in plane polar coordinates. The equation can also be used to derive the shape of the orbit for a given force law, but this usually involves the solution to a...
yields the equation of trajectory
where
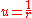 ,
, 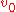 is the speed at infinity, and
is the speed at infinity, and  is the impact parameter
is the impact parameterImpact parameter
The impact parameter b is defined as the perpendicular distance between the path of a projectile and the center of the field U created by an object that the projectile is approaching...
.
The general solution of the above differential equation is
and the boundary condition is
Then we can find that
The deflection angle Θ can be seen from the graph or solving
 as
asb can be solved readily
To find the scattering cross section from this result consider its definition
Since the scattering angle is uniquely determined for a given
 and
and  , the number of particles scattered into an angle between
, the number of particles scattered into an angle between  and
and  must be the same as the number of particles with associated impact parameters between
must be the same as the number of particles with associated impact parameters between  and
and  . For an incident intensity
. For an incident intensity 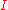 , this implies the following equality
, this implies the following equalityFor a radially symmetric scattering potential, as in the case of the Coulombic potential,
 , yielding the expression for the scattering cross section
, yielding the expression for the scattering cross sectionFinally, plugging in the previously derived expression for the impact parameter
 we find the Rutherford scattering cross section
we find the Rutherford scattering cross sectionDetails of calculating maximal nuclear size
For head on collisions between alpha particles and the nucleus, all the kinetic energyKinetic energy
The kinetic energy of an object is the energy which it possesses due to its motion.It is defined as the work needed to accelerate a body of a given mass from rest to its stated velocity. Having gained this energy during its acceleration, the body maintains this kinetic energy unless its speed changes...
of the alpha particle is turned into potential energy
Potential energy
In physics, potential energy is the energy stored in a body or in a system due to its position in a force field or due to its configuration. The SI unit of measure for energy and work is the Joule...
and the particle is at rest. The distance from the centre of the alpha particle to the centre of the nucleus (b) at this point is a maximum value for the radius, if it is evident from the experiment that the particles have not hit the nucleus.
Applying the inverse-square law
Inverse-square law
In physics, an inverse-square law is any physical law stating that a specified physical quantity or strength is inversely proportional to the square of the distance from the source of that physical quantity....
between the charges on the electron and nucleus, one can write:

Rearranging:
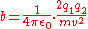
For an alpha particle:
- m (mass) = 6.7×10−27 kg
- q1 = 2×(1.6×10−19) C
- q2 (for gold) = 79×(1.6×10−19) C
- v (initial velocity) = 2×107 m/s
Substituting these in gives the value of about 2.7×10−14 m. (The true radius is about 7.3×10−15 m.) The true radius of the nucleus is not recovered in these experiments because the alphas do not have enough energy to penetrate to more than 27 fm of the nuclear center, as noted, when the actual radius of gold is 7.3 fm. Rutherford realized this, and also realized that actual impact of the alphas on gold causing any force-deviation from that of the 1/r coulomb potential would change the form of his scattering curve at high scattering angles (the smallest impact parameter
Impact parameter
The impact parameter b is defined as the perpendicular distance between the path of a projectile and the center of the field U created by an object that the projectile is approaching...
s) from a hyperbola
Hyperbola
In mathematics a hyperbola is a curve, specifically a smooth curve that lies in a plane, which can be defined either by its geometric properties or by the kinds of equations for which it is the solution set. A hyperbola has two pieces, called connected components or branches, which are mirror...
to something else. This was not seen, indicating that the gold had not been "hit" so that Rutherford only knew the gold nucleus (or the sum of the gold and alpha radii) was smaller than 27 fm (2.7×10−14 m)
Other applications
The principle of scattering is now routinely usedin Rutherford backscattering spectroscopy (RBS) to detect heavy
elements in a lower atomic number matrix, like for example heavy
metal impurities in semiconductors. In fact, the technique was also
the first local analytical technique applied on the moon, as an alpha-scattering
surface analysis instrument was operating just before
the Surveyor 4
Surveyor 4
Surveyor 4 was the fourth lunar lander in the American unmanned Surveyor program sent to explore the surface of the Moon.*Launched July 14, 1967; landed July 17, 1967*Weight on landing: 625 lb...
mission
impacted the lunar surface. The same type of instrument was
landed on the lunar surface for more leisurely period of data acquisition
on Surveyors 5 through 7.
Extension to situations with relativistic particles and target recoil
The extension of Rutherford-type scattering to energy regions in which the incoming particle has spin and magnetic moment, and is traveling at relativistic energies, and there is enough momentum-transfer that the struck particle recoils with some of the incoming particle's energy (so the process is inelastic rather than elasticElastic collision
An elastic collision is an encounter between two bodies in which the total kinetic energy of the two bodies after the encounter is equal to their total kinetic energy before the encounter...
), is called Mott scattering
Mott scattering
Mott scattering, also referred to as spin-coupling inelastic Coulomb scattering, is the separation of the two spin states of an electron beam by scattering the beam off the Coulomb field of heavy atoms...
.
External links
- E. Rutherford, The Scattering of α and β Particles by Matter and the Structure of the Atom, Philosophical Magazine. Series 6, vol. 21. May 1911
- Geiger H. & Marsden E. (1909). "On a Diffuse Reflection of the α-Particles". Proceedings of the Royal Society, Series A 82: 495-500. doi:10.1098/rspa.1909.0054.