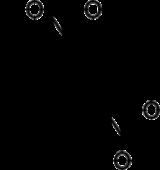
Sanger's reagent
Encyclopedia
1-fluoro-2,4-dinitrobenzene, or Sanger's reagent (commonly called dinitrofluorobenzene or DNFB), is a chemical used for polypeptide sequencing
.
(KF) in nitrobenzene
:
 In 1945, Frederick Sanger
In 1945, Frederick Sanger
described its use for determining the N-terminal amino acid
in polypeptide chains, in particular insulin
. Sanger's initial results suggested that insulin was a smaller molecule than previously estimated (molecular weight 12,000), and that it consisted of four chains (two ending in glycine
and two ending in phenylalanine
), with the chains cross-linked by disulfide bond
s. Sanger continued work on insulin, using dinitrofluorobenzene in combination with other techniques, eventually resulted in the complete sequence of insulin (consisting of only two chains, with a molecular weight of 6,000).
Following Sanger's initial report of the reagent, the dinitrofluorobenzene method was widely adopted for studying proteins, until it was superseded by other reagents for terminal analysis (e.g., dansyl chloride
and later aminopeptidase
s and carboxypeptidase
s) and other general methods for sequence determination (e.g., Edman degradation
).
Dinitrofluorobenzene reacts with the amine group in amino acids to produce dinitrophenyl-amino acids. These DNP-amino acids are moderately stable under acid hydrolysis
conditions that break peptide bond
s. The DNP-amino acids can then be recovered, and the identity of those amino acids can be discovered through chromatography
.
Protein sequencing
Protein sequencing is a technique to determine the amino acid sequence of a protein, as well as which conformation the protein adopts and the extent to which it is complexed with any non-peptide molecules...
.
Preparation
In 1936, Gottlieb presented a synthesis in which 1-chloro-2,4-dinitrobenzene reacted with potassium fluoridePotassium fluoride
Potassium fluoride is the chemical compound with the formula KF. After hydrogen fluoride, KF is the primary source of the fluoride ion for applications in manufacturing and in chemistry. It is an alkali metal halide and occurs naturally as the rare mineral carobbiite...
(KF) in nitrobenzene
Nitrobenzene
Nitrobenzene is an organic compound with the chemical formula C6H5NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale as a precursor to aniline. Although occasionally used as a flavoring or perfume...
:
Uses

Frederick Sanger
Frederick Sanger, OM, CH, CBE, FRS is an English biochemist and a two-time Nobel laureate in chemistry, the only person to have been so. In 1958 he was awarded a Nobel prize in chemistry "for his work on the structure of proteins, especially that of insulin"...
described its use for determining the N-terminal amino acid
Amino acid
Amino acids are molecules containing an amine group, a carboxylic acid group and a side-chain that varies between different amino acids. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen...
in polypeptide chains, in particular insulin
Insulin
Insulin is a hormone central to regulating carbohydrate and fat metabolism in the body. Insulin causes cells in the liver, muscle, and fat tissue to take up glucose from the blood, storing it as glycogen in the liver and muscle....
. Sanger's initial results suggested that insulin was a smaller molecule than previously estimated (molecular weight 12,000), and that it consisted of four chains (two ending in glycine
Glycine
Glycine is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its 'side chain', glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG cf. the genetic code.Glycine is a colourless, sweet-tasting crystalline solid...
and two ending in phenylalanine
Phenylalanine
Phenylalanine is an α-amino acid with the formula C6H5CH2CHCOOH. This essential amino acid is classified as nonpolar because of the hydrophobic nature of the benzyl side chain. L-Phenylalanine is an electrically neutral amino acid, one of the twenty common amino acids used to biochemically form...
), with the chains cross-linked by disulfide bond
Disulfide bond
In chemistry, a disulfide bond is a covalent bond, usually derived by the coupling of two thiol groups. The linkage is also called an SS-bond or disulfide bridge. The overall connectivity is therefore R-S-S-R. The terminology is widely used in biochemistry...
s. Sanger continued work on insulin, using dinitrofluorobenzene in combination with other techniques, eventually resulted in the complete sequence of insulin (consisting of only two chains, with a molecular weight of 6,000).
Following Sanger's initial report of the reagent, the dinitrofluorobenzene method was widely adopted for studying proteins, until it was superseded by other reagents for terminal analysis (e.g., dansyl chloride
Dansyl chloride
Dansyl chloride or 5-naphthalene-1-sulfonyl chloride is a reagent that reacts with primary amino groups in both aliphatic and aromatic amines to produce stable blue- or blue-green–fluorescent sulfonamide adducts. Dansyl chloride is widely used to modify amino acids; specifically, protein sequencing...
and later aminopeptidase
Aminopeptidase
Aminopeptidase is a zinc-dependent enzyme produced and secreted by glands of the small intestine. It helps the enzymatic digestion of proteins. Additional digestive enzymes produced by these glands include dipeptidases, maltase, sucrase, lactase, and enterokinase.Aminopeptidases catalyze the...
s and carboxypeptidase
Carboxypeptidase
A carboxypeptidase is a protease enzyme that hydrolyzes the peptide bond of an amino acid residue at the carboxy-terminal end...
s) and other general methods for sequence determination (e.g., Edman degradation
Edman degradation
Edman degradation, developed by Pehr Edman, is a method of sequencing amino acids in a peptide. In this method, the amino-terminal residue is labeled and cleaved from the peptide without disrupting the peptide bonds between other amino acid residues....
).
Dinitrofluorobenzene reacts with the amine group in amino acids to produce dinitrophenyl-amino acids. These DNP-amino acids are moderately stable under acid hydrolysis
Acid hydrolysis
Acid hydrolysis is a chemical process in which acid is used to convert cellulose or starch to sugar.It implies a chemical mechanism of hydrolysis catalyzed by a Brønsted-Lowry or Arrhenius acid. By contrast, it does not usually imply hydrolysis by direct electrophilic attack—as may originate from...
conditions that break peptide bond
Peptide bond
This article is about the peptide link found within biological molecules, such as proteins. A similar article for synthetic molecules is being created...
s. The DNP-amino acids can then be recovered, and the identity of those amino acids can be discovered through chromatography
Chromatography
Chromatography is the collective term for a set of laboratory techniques for the separation of mixtures....
.
See also
- Use of Fedric Reagent in protein sequencing
Literature
- T. Schaefer: "The Proton Magnetic Resonance Spectrum of 1-Fluoro-2,4-dinitrobenzene", Canadian Journal of ChemistryCanadian Journal of ChemistryThe Canadian Journal of Chemistry is a peer-reviewed scientific journal published by NRC Research Press. It is the official journal the Canadian Society for Chemistry...
, 1961, 40, pp. 431–433; . - B. D. Nageswara Rao: "The 1H and 19F Resonance Spectra of 1-Fluoro-2,4-dinitrobenzene", Molecular PhysicsMolecular physicsMolecular physics is the study of the physical properties of molecules, the chemical bonds between atoms as well as the molecular dynamics. Its most important experimental techniques are the various types of spectroscopy...
, 1964, 7 (4), pp. 307–310; . - A. Wilkins, R. W. H. Small: "Structure of 1-Fluoro-2,4-dinitrobenzene", Acta Cryst., 1991, C47, pp. 220–221; .

