
Sigma heat
Encyclopedia
Sigma heat, denoted 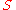 , is a is a measure of the specific energy
, is a is a measure of the specific energy
of humid air. It is used in the field of mining engineering
for calculations relating to the temperature regulation of mine air. Sigma heat is sometimes called total heat, although total heat may instead mean enthalpy
.
while simultaneously removing any condensation
formed during the process. Because sigma heat assumes that condensation will be removed, any energy which would be extracted by cooling the water vapor below its condensaton point does not count towards sigma heat. The reference temperature is usually 0 °F (-17.8 °C), although 32 °F (0 °C) is sometimes used as well.
Assuming a reference temperature of 0°F, the following formula may be used under standard temperature ranges and pressure: Different temperature ranges or pressures will slightly alter the heat capacity
of the water vapor and the air, causing a deviation from the accuracy of this formula.
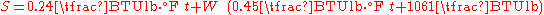
The equivalent metric formula:

of the humid air above the reference temperature. (Enthalpy is sometimes called total heat or true total heat) Unlike sigma heat, enthalpy does include the energy which would be extracted in cooling the condensed water vapor all the way to the reference temperature. Essentially, enthalpy assumes that all components of the system must be cooled during the cooling process, whereas sigma heat assumes that some of those components (liquid water) are removed part way through the process. Nevertheless, some writers mistakenly use the term enthalpy when they actually mean sigma heat, creating some confusion.
Assuming a reference temperature of 0°F, the relationship between enthalpy and sigma heat may be shown mathematically as:
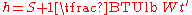
need not be taken into account unless dry bulb temperature measurements are used. Like sigma heat, the wet bulb temperature is not directly affected by the temperature of any condensed water vapor (liquid water), and it varies only when there is a net energy change to the system. In contrast, the dry bulb temperature can vary even for processes where there is no such net energy change. This difference may be understood by examining evaporative cooling. During evaporative cooling, all energy lost from air molecules as sensible heat
is gained as latent heat
by water molecules evaporating into that air. With no net energy gain
ed or lost from the now more humid air, sigma heat remains unchanged. In keeping with this, the wet bulb temperature also remains unchanged, as its reading already represented the maximum possible amount of evaporative cooling. The dry bulb temperature however is in conflict with the sigma heat since it decreases during such evaporative cooling. This is why measurements of sigma heat which use dry bulb temperatures must also take into account the humidity of the air.
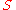 , is a is a measure of the specific energy
, is a is a measure of the specific energySpecific energy
Specific energy is defined as the energy per unit mass. Common metric units are J/kg. It is an intensive property. Contrast this with energy, which is an extensive property. There are two main types of specific energy: potential energy and specific kinetic energy. Others are the gray and sievert,...
of humid air. It is used in the field of mining engineering
Mining engineering
Mining engineering is an engineering discipline that involves the practice, the theory, the science, the technology, and application of extracting and processing minerals from a naturally occurring environment. Mining engineering also includes processing minerals for additional value.Mineral...
for calculations relating to the temperature regulation of mine air. Sigma heat is sometimes called total heat, although total heat may instead mean enthalpy
Enthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
.
Definition
Sigma heat is the energy which would be extracted from a unit mass of humid air if it were cooled to a certain reference temperature under constant pressureIsobaric process
An isobaric process is a thermodynamic process in which the pressure stays constant. The term derives from the Greek isos, , and barus,...
while simultaneously removing any condensation
Condensation
Condensation is the change of the physical state of matter from gaseous phase into liquid phase, and is the reverse of vaporization. When the transition happens from the gaseous phase into the solid phase directly, the change is called deposition....
formed during the process. Because sigma heat assumes that condensation will be removed, any energy which would be extracted by cooling the water vapor below its condensaton point does not count towards sigma heat. The reference temperature is usually 0 °F (-17.8 °C), although 32 °F (0 °C) is sometimes used as well.
Assuming a reference temperature of 0°F, the following formula may be used under standard temperature ranges and pressure: Different temperature ranges or pressures will slightly alter the heat capacity
Heat capacity
Heat capacity , or thermal capacity, is the measurable physical quantity that characterizes the amount of heat required to change a substance's temperature by a given amount...
of the water vapor and the air, causing a deviation from the accuracy of this formula.
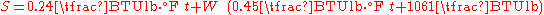
- where
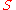 is the sigma heat of the air (in BTU/lb),
is the sigma heat of the air (in BTU/lb), is the dry bulb temperature of the air (in °F), and
is the dry bulb temperature of the air (in °F), and is the specific humidity of the air (unitless).
is the specific humidity of the air (unitless).
The equivalent metric formula:

- where
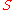 is the sigma heat of the air (in kJ/kg),
is the sigma heat of the air (in kJ/kg), is the dry bulb temperature of the air (in °C), and
is the dry bulb temperature of the air (in °C), and is the specific humidity of the air (unitless) sometimes expressed as kg/kg.
is the specific humidity of the air (unitless) sometimes expressed as kg/kg.
Comparison with enthalpy
Sigma heat is not the same as the enthalpyEnthalpy
Enthalpy is a measure of the total energy of a thermodynamic system. It includes the internal energy, which is the energy required to create a system, and the amount of energy required to make room for it by displacing its environment and establishing its volume and pressure.Enthalpy is a...
of the humid air above the reference temperature. (Enthalpy is sometimes called total heat or true total heat) Unlike sigma heat, enthalpy does include the energy which would be extracted in cooling the condensed water vapor all the way to the reference temperature. Essentially, enthalpy assumes that all components of the system must be cooled during the cooling process, whereas sigma heat assumes that some of those components (liquid water) are removed part way through the process. Nevertheless, some writers mistakenly use the term enthalpy when they actually mean sigma heat, creating some confusion.
Assuming a reference temperature of 0°F, the relationship between enthalpy and sigma heat may be shown mathematically as:
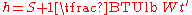
- where
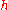 is the specific enthalpy of the air above its reference temperature,
is the specific enthalpy of the air above its reference temperature,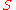 is the sigma heat of the air (in BTU/lb),
is the sigma heat of the air (in BTU/lb), is the specific humidity of the air (unitless), and
is the specific humidity of the air (unitless), and is the wet bulb temperature (in °F).
is the wet bulb temperature (in °F).
Wet bulb temperature vs. dry bulb temperature
Assuming constant pressure, sigma heat is solely a function of the wet bulb temperature of the air. For this reason, humidityHumidity
Humidity is a term for the amount of water vapor in the air, and can refer to any one of several measurements of humidity. Formally, humid air is not "moist air" but a mixture of water vapor and other constituents of air, and humidity is defined in terms of the water content of this mixture,...
need not be taken into account unless dry bulb temperature measurements are used. Like sigma heat, the wet bulb temperature is not directly affected by the temperature of any condensed water vapor (liquid water), and it varies only when there is a net energy change to the system. In contrast, the dry bulb temperature can vary even for processes where there is no such net energy change. This difference may be understood by examining evaporative cooling. During evaporative cooling, all energy lost from air molecules as sensible heat
Sensible heat
Sensible heat is the energy exchanged by a thermodynamic system that has as its sole effect a change of temperature.The term is used in contrast to a latent heat, which is the amount of energy exchanged that is hidden, meaning it cannot be observed as a change of temperature...
is gained as latent heat
Latent heat
Latent heat is the heat released or absorbed by a chemical substance or a thermodynamic system during a process that occurs without a change in temperature. A typical example is a change of state of matter, meaning a phase transition such as the melting of ice or the boiling of water. The term was...
by water molecules evaporating into that air. With no net energy gain
Net energy gain
Net Energy Gain is a concept used in energy economics that refers to the difference between the energy expended to harvest an energy source and the amount of energy gained from that harvest. The net energy gain, which can be expressed in joules, differs from the net financial gain that may result...
ed or lost from the now more humid air, sigma heat remains unchanged. In keeping with this, the wet bulb temperature also remains unchanged, as its reading already represented the maximum possible amount of evaporative cooling. The dry bulb temperature however is in conflict with the sigma heat since it decreases during such evaporative cooling. This is why measurements of sigma heat which use dry bulb temperatures must also take into account the humidity of the air.

