
Tellurate
Encyclopedia
Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
is TeO42− or TeO66−.
Unlike sulfate, tellurate is a somewhat good oxidizer; it can be reduced to tellurite
Tellurite (ion)
The tellurite ion is TeO32−. A tellurite is a compound that contains this ion. An example is sodium tellurite. Tellurites are one of the more stable tellurium compounds, although they can be reduced to elemental tellurium by electrolysis or a strong reducing agent.-Acidified forms:In...
or tellurium.
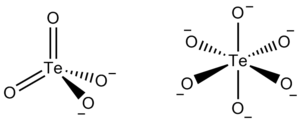
Tellurate exists in two forms, metatellurate ion, TeO42−, and orthotellurate ion, TeO66−.
Compounds include metatellurates and orthotellurates. Metatellurates are analogous to sulfate
Sulfate
In inorganic chemistry, a sulfate is a salt of sulfuric acid.-Chemical properties:...
s, but they are rare. Orthotellurates are much more common and this forms most of the chemistry of tellurates.
In neutral conditions, pentahydrogen orthotellurate ion, H5TeO6−, is most common; in basic conditions, tetrahydrogen orthotellurate ion, H4TeO62−, is found; in acid conditions, the orthotelluric acid
Telluric acid
Telluric acid is a chemical compound with the formula Te6. It is a white solid made up of octahedral Te6 molecules which persist in aqueous solution...
, H6TeO6 is formed.
Properties
The metatellurate anion, TeO42−, has a molecular weight of 191.60 g/mole. It belongs to the Td molecular point group, thus it has a tetrahedral shape even though two of its oxygen atoms are double bonded.- TeO42− → TeO32− + 1/2O2 (E0= −1.042 V)
The E0 or standard reduction potential value is significant as it gives an indication of the strength of the tellurate ion as an oxidizing agent.
The orthotellurate anion, TeO66−, has a molecular weight of 223.6 g/mole. It belongs to the Oh molecular point group, thus it has an octahedral shape.
NMR spectroscopy
Tellurium has two NMR active nuclei, 123Te and 125Te. 123Te has an abundance of 0.9% and a nuclear spin (I) of 1/2. 125Te has an abundance of 7% and an equivalent nuclear spin. 125Te is more commonly performed because it has a higher sensitivity. The metatellurate anion has a chemical shiftChemical shift
In nuclear magnetic resonance spectroscopy, the chemical shift is the resonant frequency of a nucleus relative to a standard. Often the position and number of chemical shifts are diagnostic of the structure of a molecule...
around 610 ppm when analyzed using 125Te NMR at 25°C at a frequency of 94.735 MHz and referenced externally against aqueous 1.0 M telluric acid.
List of some known salts involving metatellurate anions
Na2TeO4*2H2OK2TeO4*2H2O
Rb2TeO4
Cs2TeO4
CaTeO4
Al2(TeO4)3
(NH4)2TeO4

