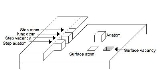
Terrace ledge kink
Encyclopedia
The Terrace Ledge Kink model, which is also referred to as the Terrace Step Kink (TSK) model, describes the thermodynamics of crystal
surface formation and transformation, as well as the energetics of surface defect formation. It is based upon the idea that the energy of an atom’s position on a crystal surface is determined by its bonding to neighboring atoms and that transitions simply involve the counting of broken and formed bonds. The TLK model can be applied to surface science
topics such as crystal growth
, surface diffusion
, roughening, and vaporization
.
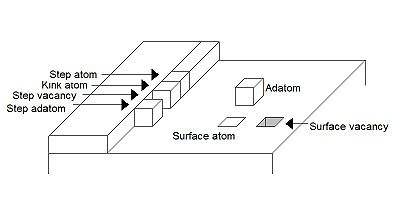
 Depending on the position of an atom on a surface, it can be referred to by one of several names. Figure 1 illustrates the names for the atomic positions and point defects on a surface for a simple cubic lattice.
Depending on the position of an atom on a surface, it can be referred to by one of several names. Figure 1 illustrates the names for the atomic positions and point defects on a surface for a simple cubic lattice.
Figure 2 shows a scanning tunneling microscopy topographic image of a step edge that shows many of the features in Figure 1.
of 6 nearest neighbors. Second-nearest neighbors in this cubic model are those that share an edge and third-nearest neighbors are those that share corners. The number of neighbors, second-nearest neighbors, and third-nearest neighbors for each of the different atom positions are given in Table 1.
Most crystals, however, are not arranged in a simple cubic lattice. The same ideas apply for other types of lattices where the coordination number is not six, but these are not as easy to visualize and work with in theory, so the remainder of the discussion will focus on simple cubic lattices. Table 2 indicates the number of neighboring atoms for a bulk atom in some other crystal lattices.
The kink site is of special importance when evaluating the thermodynamics
of a variety of phenomena. This site is also referred to as the “half-crystal position” and energies are evaluated relative to this position for processes such as adsorption, surface diffusion, and sublimation. The term “half-crystal” comes from the fact that the kink site has half the number of neighboring atoms as an atom in the crystal bulk, regardless of the type of crystal lattice.
For example, the formation energy for an adatom—ignoring any crystal relaxation—is calculated by subtracting the energy of an adatom from the energy of the kink atom.
This can be understood as the breaking of all of the kink atom’s bonds to remove the atom from the surface and then reforming the adatom interactions. This is equivalent to a kink atom diffusing away from the rest of the step to become a step adatom and then diffusing away from the adjacent step onto the terrace to become an adatom. In the case where all interactions are ignored except for those with nearest neighbors, the formation energy for an adatom would be the following, where is the bond energy in the crystal is given by Equation 2.
is the bond energy in the crystal is given by Equation 2.
This can be extended to a variety of situations, such as the formation of an adatom-surface vacancy pair on a terrace, which would involve the removal of a surface atom from the crystal and placing it as an adatom on the terrace. This is described by Equation 3.
The energy of sublimation would simply be the energy required to remove an atom from the kink site. This can be envisioned as the surface being disassembled one terrace at a time by removing atoms from the edge of each step, which is the kink position. It has been demonstrated that the application of an external electric field
will induce the formation of additional kinks in a surface, which then leads to a faster rate of evaporation from the surface.
is described by equation 4, where n0 is the total number of surface sites per unit area:
This can be extended to find the equilibrium concentration of other types of surface point defects as well. To do so, the energy of the defect in question is simply substituted into the above equation in the place of the energy of adatom formation.
Crystal
A crystal or crystalline solid is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. The scientific study of crystals and crystal formation is known as crystallography...
surface formation and transformation, as well as the energetics of surface defect formation. It is based upon the idea that the energy of an atom’s position on a crystal surface is determined by its bonding to neighboring atoms and that transitions simply involve the counting of broken and formed bonds. The TLK model can be applied to surface science
Surface science
Surface science is the study of physical and chemical phenomena that occur at the interface of two phases, including solid–liquid interfaces, solid–gas interfaces, solid–vacuum interfaces, and liquid-gas interfaces. It includes the fields of surface chemistry and surface physics. Some related...
topics such as crystal growth
Crystal growth
A crystal is a solid material whose constituent atoms, molecules, or ions are arranged in an orderly repeating pattern extending in all three spatial dimensions. Crystal growth is a major stage of a crystallization process, and consists in the addition of new atoms, ions, or polymer strings into...
, surface diffusion
Surface diffusion
Surface diffusion is a general process involving the motion of adatoms, molecules, and atomic clusters at solid material surfaces. The process can generally be thought of in terms of particles jumping between adjacent adsorption sites on a surface, as in figure 1...
, roughening, and vaporization
Vaporization
Vaporization of an element or compound is a phase transition from the liquid or solid phase to gas phase. There are three types of vaporization: evaporation, boiling and sublimation....
.
History
The TLK model is credited as having originated out of two German papers by Kossel and Stranski wherein the thermodynamic stability of step edges were discussed.Definitions


Figure 2 shows a scanning tunneling microscopy topographic image of a step edge that shows many of the features in Figure 1.
Thermodynamics
The energy required to remove an atom from the surface depends on the number of bonds to other surface atoms which must be broken. For a simple cubic lattice in this model, each atom is treated as a cube and bonding occurs at each face, giving a coordination numberCoordination number
In chemistry and crystallography, the coordination number of a central atom in a molecule or crystal is the number of its nearest neighbours. This number is determined somewhat differently for molecules and for crystals....
of 6 nearest neighbors. Second-nearest neighbors in this cubic model are those that share an edge and third-nearest neighbors are those that share corners. The number of neighbors, second-nearest neighbors, and third-nearest neighbors for each of the different atom positions are given in Table 1.
| Atom | Nearest Neighbors | Second-Nearest Neighbors | Third-Nearest Neighbors |
|---|---|---|---|
| Adatom | 1 | 4 | 4 |
| Step adatom | 2 | 6 | 4 |
| Kink atom | 3 | 6 | 4 |
| Step atom | 4 | 6 | 4 |
| Surface atom | 5 | 8 | 4 |
| Bulk atom | 6 | 12 | 8 |
Most crystals, however, are not arranged in a simple cubic lattice. The same ideas apply for other types of lattices where the coordination number is not six, but these are not as easy to visualize and work with in theory, so the remainder of the discussion will focus on simple cubic lattices. Table 2 indicates the number of neighboring atoms for a bulk atom in some other crystal lattices.
| Lattice | Nearest Neighbors | Second-Nearest Neighbors | Third-Nearest Neighbors |
|---|---|---|---|
| Simple cubic | 6 | 12 | 8 |
| Face-centered cubic | 12 | 6 | 24 |
| Body-centered cubic | 8 | 6 | 12 |
| Hexagonal close packed | 12 | 6 | 2 |
| Diamond Diamond In mineralogy, diamond is an allotrope of carbon, where the carbon atoms are arranged in a variation of the face-centered cubic crystal structure called a diamond lattice. Diamond is less stable than graphite, but the conversion rate from diamond to graphite is negligible at ambient conditions... |
4 | 12 | 12 |
The kink site is of special importance when evaluating the thermodynamics
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
of a variety of phenomena. This site is also referred to as the “half-crystal position” and energies are evaluated relative to this position for processes such as adsorption, surface diffusion, and sublimation. The term “half-crystal” comes from the fact that the kink site has half the number of neighboring atoms as an atom in the crystal bulk, regardless of the type of crystal lattice.
For example, the formation energy for an adatom—ignoring any crystal relaxation—is calculated by subtracting the energy of an adatom from the energy of the kink atom.
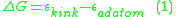 |
This can be understood as the breaking of all of the kink atom’s bonds to remove the atom from the surface and then reforming the adatom interactions. This is equivalent to a kink atom diffusing away from the rest of the step to become a step adatom and then diffusing away from the adjacent step onto the terrace to become an adatom. In the case where all interactions are ignored except for those with nearest neighbors, the formation energy for an adatom would be the following, where
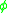 is the bond energy in the crystal is given by Equation 2.
is the bond energy in the crystal is given by Equation 2. 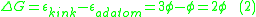 |
This can be extended to a variety of situations, such as the formation of an adatom-surface vacancy pair on a terrace, which would involve the removal of a surface atom from the crystal and placing it as an adatom on the terrace. This is described by Equation 3.
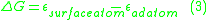 |
The energy of sublimation would simply be the energy required to remove an atom from the kink site. This can be envisioned as the surface being disassembled one terrace at a time by removing atoms from the edge of each step, which is the kink position. It has been demonstrated that the application of an external electric field
Electric field
In physics, an electric field surrounds electrically charged particles and time-varying magnetic fields. The electric field depicts the force exerted on other electrically charged objects by the electrically charged particle the field is surrounding...
will induce the formation of additional kinks in a surface, which then leads to a faster rate of evaporation from the surface.
Temperature dependence of defect coverage
The number of adatoms present on a surface is temperature dependent. The relationship between the surface adatom concentration and the temperature at equilibriumThermodynamic equilibrium
In thermodynamics, a thermodynamic system is said to be in thermodynamic equilibrium when it is in thermal equilibrium, mechanical equilibrium, radiative equilibrium, and chemical equilibrium. The word equilibrium means a state of balance...
is described by equation 4, where n0 is the total number of surface sites per unit area:
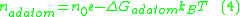 |
This can be extended to find the equilibrium concentration of other types of surface point defects as well. To do so, the energy of the defect in question is simply substituted into the above equation in the place of the energy of adatom formation.

