Tetrarhodium dodecacarbonyl
Encyclopedia
Tetrarhodium dodecacarbonyl is the chemical compound
with the formula
Rh4(CO)12. This dark-red crystalline solid is the smallest stable binary rhodium carbonyl. It is used as a catalyst in organic synthesis
.
It is prepared by treatment of an aqueous solution of rhodium trichloride with activated copper
metal under an atmosphere of CO.
Alternatively, the compound can be prepared by treatment of a methanolic solution of RhCl3(H2O)3 with CO to afford H[RhCl2(CO)2], followed by carbonylation in the presence of sodium citrate
.
The cluster undergoes thermal substitution
with phosphorus ligands:
catalysis, the metal carbonyl
s has been systematically studied to a high degree. The instability of Rh2(CO)8 has been a source of curiosity. The analogous binary carbonyl of cobalt, Co2(CO)8, is well known. Solutions of Rh5(CO)12 under high pressures of CO do convert to the dirhodium compound:
The relative instability of Rh2(CO)8 conforms with a general trend: Ru(CO)5 loses CO spontaneously to give Ru3(CO)12.
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
with the formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
Rh4(CO)12. This dark-red crystalline solid is the smallest stable binary rhodium carbonyl. It is used as a catalyst in organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
.
Structure, synthesis, reactions
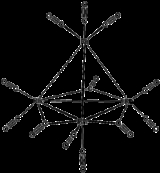
The structure of Rh4(CO)12 is described by a tetrahedral array of four Rh atoms with nine terminal CO ligands and three bridging CO ligands. The structure can be expressed as Rh4(CO)9(µ-CO)3.It is prepared by treatment of an aqueous solution of rhodium trichloride with activated copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
metal under an atmosphere of CO.
- 4 RhCl3(H2O)3 + 8 Cu + 22 CO → Rh4(CO)12 + 2 CO2 + 8 Cu(CO)Cl + 4 HCl + 10 H2O
Alternatively, the compound can be prepared by treatment of a methanolic solution of RhCl3(H2O)3 with CO to afford H[RhCl2(CO)2], followed by carbonylation in the presence of sodium citrate
Sodium citrate
Trisodium citrate has the chemical formula of Na3C6H5O7. It is sometimes referred to simply as sodium citrate, though sodium citrate can refer to any of the three sodium salts of citric acid. It possesses a saline, mildly tart flavor. For this reason, citrates of certain alkaline and alkaline earth...
.
The cluster undergoes thermal substitution
Substitution reaction
In a substitution reaction, a functional group in a particular chemical compound is replaced by another group. In organic chemistry, the electrophilic and nucleophilic substitution reactions are of prime importance...
with phosphorus ligands:
- Rh4(CO)12-n + n L → Rh4(CO)12-nLn + n CO
Related metal carbonyls
Because of their relevance to hydroformylationHydroformylation
Hydroformylation, also known as oxo synthesis or oxo process, is an important industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group and a hydrogen atom to a carbon-carbon double bond...
catalysis, the metal carbonyl
Metal carbonyl
Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. These complexes may be homoleptic, that is containing only CO ligands, such as nickel carbonyl , but more commonly metal carbonyls contain a mix of ligands, such as Re3Cl...
s has been systematically studied to a high degree. The instability of Rh2(CO)8 has been a source of curiosity. The analogous binary carbonyl of cobalt, Co2(CO)8, is well known. Solutions of Rh5(CO)12 under high pressures of CO do convert to the dirhodium compound:
- Rh4(CO)12 + 4 CO → 2 Rh2(CO)8
The relative instability of Rh2(CO)8 conforms with a general trend: Ru(CO)5 loses CO spontaneously to give Ru3(CO)12.
General reading
- King, R. B., "Rhodium: Organometallic Chemistry" Encyclopedia of Inorganic Chemistry 1994, 7, 3494.

