Thymocyte
Encyclopedia
Thymocytes are hematopoietic progenitor cells present in the thymus
. Thymopoiesis is the process in the thymus by which thymocytes differentiate into mature T lymphocytes. The primary function of thymocytes is the generation of T lymphocytes (T cells). The thymus provides an inductive environment, which allows for the development and selection of physiologically useful T cells. The processes of beta-selection, positive selection, and negative selection shape the population thymocytes into a peripheral pool of T cells that are able to respond to foreign pathogens and are immunologically tolerant towards self antigens.
) reside in bone marrow. They produce precursors of T lymphocytes, which seed the thymus
(thus becoming thymocytes) and differentiate under influence of the Notch
and its ligands.
Early, double negative thymocytes express (and can be identified by) CD2
, CD5
and CD7
. Still during the double negative stage, CD34
expression stops and CD1
is expressed. Expression of both CD4 and CD8 makes them double positive, and matures into either CD4+ or CD8+ cells.
(CD62P), and the chemokine receptors CCR7 and CCR9
.
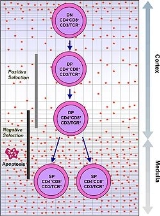
Following thymus entry, progenitors proliferate to generate the ETP population. This step is following by the generation of DN2 thymocytes which migrate from the cortico-medullary junction toward the thymus capsule. DN3 thymocytes are generated at the subcapsular zone.
In addition to proliferation, differentiation and T lineage commitment occurs within the DN thymocyte population. Commitment, or loss of alternative lineage potentials (such as myeloid, B, and NK lineage potentials), is dependent on Notch signaling
, and is complete by the DN3 stage. Following T lineage commitment, DN3 thymocytes undergo β-selection.

 The ability of T cells to recognize foreign antigens is mediated by the T cell receptor
The ability of T cells to recognize foreign antigens is mediated by the T cell receptor
(TCR), which is a surface protein able to recognise short protein sequences (peptides) that are presented on MHC
. The purpose of thymocyte development is to produce mature T cells with a diverse array of functional T cell receptors, through the process of TCR gene rearrangement.
Unlike most genes, which have a stable sequence in each cell which expresses them, the T cell receptor
is made up of a series of alternative gene fragments. In order to create a functional T cell receptor, the double negative thymocytes use a series of DNA-interacting enzymes to clip the DNA and bring separate gene fragments together. The outcome of this process is that each T cell receptor
has a different sequence, due to different choice of gene fragments and the errors introduced during the cutting and joining process (see section on V(D)J recombination
for more information on TCR rearrangement). The evolutionary advantage in having a large number of unique T cell receptor
s is that each T cell is capable of recognizing a different peptide, providing a defense against rapidly evolving pathogens.
TCR rearrangement occurs in two steps. First the TCRβ chain is rearranged at the DN3 stage of T cell development. The TCRβ chain is paired with the pre-Tα to generate the pre-TCR. The cellular disadvantage in the rearrangement process is that many of the combinations of the T cell receptor
gene fragments are non-functional. To eliminate thymocytes which have made a non-functional T cell receptor
, only cells that have successfully rearranged the beta chain to produce a functional pre-TCR are allowed to develop beyond the DN3 stage. Cells that fail to produce a functional pre-TCR are eliminated by apoptosis
. This process is referred to as the beta-selection checkpoint. Successful beta-selection requires that TCRβ is produced, TCRβ is capable of pairing with pre-Tα to generate the pre-TCR, and that the pre-TCR can interact on the cell surface with the TCR signalling proteins.
Following β-selection thymocytes generate CD4+CD8+ double positive cells, which then undergo TCRα rearrangement, resulting in completely assembled TCR.
which is capable of assembling on the surface. However many of these T cell receptor
s will still be non-functional, due to an inability to bind MHC
. The next major stage of thymocyte development is positive selection, to keep only those thymocytes which have a T cell receptor capable of binding MHC
. The T cell receptor requires CD8 as a coreceptor to bind to MHC
class I, and CD4
as a coreceptor to bind MHC
class II. At this stage thymocytes upregulate both CD4
and CD8
, becoming double positive cells.
Double positive thymocytes that have a T cell receptor capable of binding MHC class I or class II (even with a weak affinity) receive signalling through the T cell receptor. Thymocytes that have a T cell receptor incapable of binding MHC class I or class II undergo apoptosis
. Some thymocytes are able to rescue failed positive selection by receptor editing (rearrangement of the other T cell receptor allele to produce a new T cell receptor).
The double positive thymocytes undergo lineage commitment, maturing into a CD8+ T cell (recognising MHC class I) or a CD4+ T cell (recognising MHC class II). Lineage commitment occurs at the late stage of positive selection and works by downregulation of both CD4 and CD8 (reducing the signal from the T cell receptor) and then upregulation of CD4 only. Thymocytes that start receiving signal again are those that recognise MHC class II, and they become CD4+ T cells. Thymocytes that do not start receiving signal again are those that recognize MHC class I, and they downregulate CD4 and upregulate CD8, to become CD8+ T cells. Both of these thymocytes types are known as single positive thymocytes.
receptor CCR7, causing migration from the cortex to the medulla. At this stage the key maturation process involves negative selection, the elimination of autoreactive thymocytes.
The key disadvantage in a gene rearrangement process for T cell receptor
s is that by random chance, some arrangements of gene fragments will create a T cell receptor capable of binding self-peptides presented on MHC class I or MHC class II. If T cells bearing these T cell receptors were to enter the periphery, they would be capable of activating an immune response against self, resulting in autoimmunity
. Negative selection is the process evolved to reduce this risk. During negative selection, all thymocytes with a high affinity for binding self peptides presented on MHC class I or class II are induced to upregulate Bim
, a protein which drives apoptosis. Cells which do not have a high affinity for self survive negative selection. At this stage, some cells are also selected to become regulatory T cells, usually cells which have an intermediate affinity for self-peptide.
Negative selection can occur at the double positive stage in the cortex. However the repertoire of peptides in the cortex is limited to those expressed by epithelial cells, and double positive cells are poor at undergoing negative selection. Therefore the most important site for negative selection is the medulla, once cells are at the single positive stage. In order to remove thymocytes reactive to peripheral organs, the transcription factor AIRE
drives the expression of multiple peripheral antigens, such as insulin, resulting in deletion of cells specific for those antigens. This allows single positive thymocytes to be exposed to a more complex set of self-antigens than is present in the cortex, and therefore more efficiently deletes those T cells which are autoreactive.
Single positive thymocytes remain in the medulla for 1–2 weeks, surveying self-antigens to test for autoreactivity. During this time they undergo final maturational changes, and then exit the thymus using S1P and CCR7. Upon entry to the peripheral bloodstream, the cells are considered mature T cells, and not thymocytes.
Negative selection is not 100% complete, some autoreactive T cells escape thymic censorship, and are released into the circulation. Additional mechanisms of peripheral tolerance
active in the periphery exist to silence these cells such as anergy
, deletion, and regulatory T cells. If these peripheral tolerance mechanisms also fail, autoimmunity
may arise.
Thymus transplantation
results in that T cells are taught to avoid reacting with donor antigens instead, and may still react with many self-antigens in the body. Autoimmune disease is a frequent complication after thymus transplantation, found in 42% of subjects over 1 year post-transplantation. However, this is partially explained by that the indication itself, that is, complete DiGeorge syndrome
(absence of thymus), increases the risk of autoimmune disease.
Thymus
The thymus is a specialized organ of the immune system. The thymus produces and "educates" T-lymphocytes , which are critical cells of the adaptive immune system....
. Thymopoiesis is the process in the thymus by which thymocytes differentiate into mature T lymphocytes. The primary function of thymocytes is the generation of T lymphocytes (T cells). The thymus provides an inductive environment, which allows for the development and selection of physiologically useful T cells. The processes of beta-selection, positive selection, and negative selection shape the population thymocytes into a peripheral pool of T cells that are able to respond to foreign pathogens and are immunologically tolerant towards self antigens.
Stages of maturation
Thymocytes are classified into a number of distinct maturational stages based on the expression of cell surface markers. The earliest thymocyte stage is the double negative stage (negative for both CD4 and CD8), which more recently has been better described as Lineage-negative, and which can be divided into four substages. The next major stage is the double positive stage (positive for both CD4 and CD8). The final stage in maturation is the single positive stage (positive for either CD4 or CD8).In mice
| Stage | Defining surface markers | Location | Significant events >- | Double negative 1 or ETP (Early T lineage Progenitor) |
Lineage-CD44 CD44 The CD44 antigen is a cell-surface glycoprotein involved in cell–cell interactions, cell adhesion and migration. In humans, the CD44 antigen is encoded by the CD44 gene.- Tissue distribution and isoforms :... +CD25 CD25 CD25 is the alpha chain of the IL-2 receptor. It is a type I transmembrane protein present on activated T cells, activated B cells, some thymocytes, myeloid precursors, and oligodendrocytes that associates with CD122 to form a heterodimer that can act as a high-affinity receptor for IL-2.CD25 is... -CD117 CD117 Mast/stem cell growth factor receptor also known as proto-oncogene c-Kit or tyrosine-protein kinase Kit or CD117 is a protein that in humans is encoded by the KIT gene... + |
cortex | >- | Lineage-CD44+CD25+CD117+ | cortex | >- | Lineage-CD44-CD25+ | cortex | >- | Lineage-CD44-CD25- | cortex | >- | CD4 CD4 CD4 is a glycoprotein expressed on the surface of T helper cells, monocytes, macrophages, and dendritic cells. It was discovered in the late 1970s and was originally known as leu-3 and T4 before being named CD4 in 1984... +CD8 CD8 CD8 is a transmembrane glycoprotein that serves as a co-receptor for the T cell receptor . Like the TCR, CD8 binds to a major histocompatibility complex molecule, but is specific for the class I MHC protein. There are two isoforms of the protein, alpha and beta, each encoded by a different gene... + |
cortex | >- | CD4+CD8- or CD4-CD8+ | medulla | Negative selection |
In humans
In human, circulating pluripotent CD34+ hematopoietic stem cells (HSCHSC
-Computing:* Hitachi Storage Cluster* HSC50 , a mass storage controller from Digital Equipment Corporation implementing their Mass Storage Control Protocol* Hughes Systique Corporation-Medicine:...
) reside in bone marrow. They produce precursors of T lymphocytes, which seed the thymus
Thymus
The thymus is a specialized organ of the immune system. The thymus produces and "educates" T-lymphocytes , which are critical cells of the adaptive immune system....
(thus becoming thymocytes) and differentiate under influence of the Notch
Notch
Notch may refer to:* The nock of an arrow* Notch , a Hip hop, R&B, reggae, dancehall and reggaeton artist* Notch signaling pathway, a cell signaling system present in most multicellular organisms...
and its ligands.
Early, double negative thymocytes express (and can be identified by) CD2
CD2
CD2 is a cell adhesion molecule found on the surface of T cells and natural killer cells.It has also been called T-cell surface antigen T11/Leu-5, LFA-2, LFA-3 receptor, erythrocyte receptor and rosette receptor....
, CD5
CD5
CD5 or CD-5 may be:* Child development-5, for five-year old children, a more developmentally appropriate acronym for kindergarten* cluster of differentiation 5 molecule, type I transmembrane protein* compact disc, 5-inch CD, usually music CD...
and CD7
CD7
CD7 is a human protein encoded by the gene.-External links:...
. Still during the double negative stage, CD34
CD34
CD34 molecule is a cluster of differentiation molecule present on certain cells within the human body. It is a cell surface glycoprotein and functions as a cell-cell adhesion factor. It may also mediate the attachment of stem cells to bone marrow extracellular matrix or directly to stromal cells...
expression stops and CD1
CD1
For the album by Throbbing Gristle, see CD1 CD1 is a family of glycoproteins expressed on the surface of various human antigen-presenting cells. They are related to the class I MHC molecules, and are involved in the presentation of lipid antigens to T cells...
is expressed. Expression of both CD4 and CD8 makes them double positive, and matures into either CD4+ or CD8+ cells.
Events during maturation
| type: | functional (beta selection) | functional (positive selection) | autoreactive (negative selection) >- | location: |
cortex | cortex | >- | In order to pass the β-selection checkpoint, the β chain of the T cell receptor T cell receptor The T cell receptor or TCR is a molecule found on the surface of T lymphocytes that is responsible for recognizing antigens bound to major histocompatibility complex molecules... rearranged by the thymocyte must retain the structural properties allowing it to be presented on the surface of the thymocyte with pre-TCRα. This eliminates thymocytes with gross defects introduced into the T cell receptor T cell receptor The T cell receptor or TCR is a molecule found on the surface of T lymphocytes that is responsible for recognizing antigens bound to major histocompatibility complex molecules... by gene rearrangement. |
In order to be positively-selected, thymocytes will have to interact with several cell surface molecules, MHC MHC -Biology:*Myosin heavy chain - part of the motor protein myosin's quaternary protein structure*Major histocompatibility complex - a highly polymorphic region on chromosome 6 with genes particularly involved in immune functions-Colleges:... , to ensure reactivity and specificity. Positive selection selects cells with a T cell receptor T cell receptor The T cell receptor or TCR is a molecule found on the surface of T lymphocytes that is responsible for recognizing antigens bound to major histocompatibility complex molecules... able to bind MHC Major histocompatibility complex Major histocompatibility complex is a cell surface molecule encoded by a large gene family in all vertebrates. MHC molecules mediate interactions of leukocytes, also called white blood cells , which are immune cells, with other leukocytes or body cells... class I or II molecules with at least a weak affinity. This eliminates (by a process called "death by neglect") those T cells which would be non-functional due to an inability to bind MHC. |
Negative selection is the active induction of apoptosis in thymocytes with a high affinity for self peptides or MHC. This eliminates cells which would direct immune responses towards self-proteins in the periphery. Negative selection is not 100% complete, some autoreactive T cells escape thymic censorship, and are released into the circulation. Additional mechanisms of tolerance active in the periphery exist to silence these cells such as anergy Anergy Anergy is a term in immunobiology that describes a lack of reaction by the body's defense mechanisms to foreign substances, and consists of a direct induction of peripheral lymphocyte tolerance. An individual in a state of anergy often indicates that the immune system is unable to mount a normal... , deletion, and regulatory T cells. If these peripheral tolerance Peripheral tolerance Peripheral tolerance is immunological tolerance developed after T and B cells mature and enter the periphery. These include the suppression of autoreactive cells by 'regulatory' T cells and the generation of hyporesponsiveness in lymphocytes which encounter antigen in the absence of the... mechanisms also fail, autoimmunity Autoimmunity Autoimmunity is the failure of an organism to recognize its own constituent parts as self, which allows an immune response against its own cells and tissues. Any disease that results from such an aberrant immune response is termed an autoimmune disease... may arise. |
Thymus settling
Thymocytes are ultimately derived from bone marrow hematopoietic progenitors cells [see hematopoietic stem cell, hematopoiesis] which reach the thymus through the circulation. The number of progenitors that enter the thymus each day is thought to be extremely small. Therefore which progenitors colonize the thymus is unknown. Currently Early Lymphoid Progenitors (ELP) are proposed to settle the thymus and are likely the precursors of at least some thymocytes. ELPs are Lineage-CD44+CD25-CD117+ and thus closely resemble ETPs, the earliest progenitors in the thymus. Precursors enter the thymus at the cortico-medullary junction. Molecules known to be important for thymus entry include P-selectinP-selectin
P-selectin is a cell adhesion molecule on the surfaces of activated endothelial cells, which line the inner surface of blood vessels, and activated platelets...
(CD62P), and the chemokine receptors CCR7 and CCR9
CCR9
C-C chemokine receptor type 9 is a protein that in humans is encoded by the CCR9 gene.CCR9 has also recently been designated CDw199 .-Further reading:...
.
Following thymus entry, progenitors proliferate to generate the ETP population. This step is following by the generation of DN2 thymocytes which migrate from the cortico-medullary junction toward the thymus capsule. DN3 thymocytes are generated at the subcapsular zone.
In addition to proliferation, differentiation and T lineage commitment occurs within the DN thymocyte population. Commitment, or loss of alternative lineage potentials (such as myeloid, B, and NK lineage potentials), is dependent on Notch signaling
Notch signaling
The notch signaling pathway is a highly conserved cell signaling system present in most multicellular organisms.Notch is present in all metazoans, and mammals possess four different notch receptors, referred to as NOTCH1, NOTCH2, NOTCH3, and NOTCH4. The notch receptor is a single-pass...
, and is complete by the DN3 stage. Following T lineage commitment, DN3 thymocytes undergo β-selection.
β-selection


T cell receptor
The T cell receptor or TCR is a molecule found on the surface of T lymphocytes that is responsible for recognizing antigens bound to major histocompatibility complex molecules...
(TCR), which is a surface protein able to recognise short protein sequences (peptides) that are presented on MHC
Major histocompatibility complex
Major histocompatibility complex is a cell surface molecule encoded by a large gene family in all vertebrates. MHC molecules mediate interactions of leukocytes, also called white blood cells , which are immune cells, with other leukocytes or body cells...
. The purpose of thymocyte development is to produce mature T cells with a diverse array of functional T cell receptors, through the process of TCR gene rearrangement.
Unlike most genes, which have a stable sequence in each cell which expresses them, the T cell receptor
T cell receptor
The T cell receptor or TCR is a molecule found on the surface of T lymphocytes that is responsible for recognizing antigens bound to major histocompatibility complex molecules...
is made up of a series of alternative gene fragments. In order to create a functional T cell receptor, the double negative thymocytes use a series of DNA-interacting enzymes to clip the DNA and bring separate gene fragments together. The outcome of this process is that each T cell receptor
T cell receptor
The T cell receptor or TCR is a molecule found on the surface of T lymphocytes that is responsible for recognizing antigens bound to major histocompatibility complex molecules...
has a different sequence, due to different choice of gene fragments and the errors introduced during the cutting and joining process (see section on V(D)J recombination
V(D)J recombination
VJ recombination, also known as somatic recombination, is a mechanism of genetic recombination in the early stages of immunoglobulin and T cell receptors production of the immune system...
for more information on TCR rearrangement). The evolutionary advantage in having a large number of unique T cell receptor
T cell receptor
The T cell receptor or TCR is a molecule found on the surface of T lymphocytes that is responsible for recognizing antigens bound to major histocompatibility complex molecules...
s is that each T cell is capable of recognizing a different peptide, providing a defense against rapidly evolving pathogens.
TCR rearrangement occurs in two steps. First the TCRβ chain is rearranged at the DN3 stage of T cell development. The TCRβ chain is paired with the pre-Tα to generate the pre-TCR. The cellular disadvantage in the rearrangement process is that many of the combinations of the T cell receptor
T cell receptor
The T cell receptor or TCR is a molecule found on the surface of T lymphocytes that is responsible for recognizing antigens bound to major histocompatibility complex molecules...
gene fragments are non-functional. To eliminate thymocytes which have made a non-functional T cell receptor
T cell receptor
The T cell receptor or TCR is a molecule found on the surface of T lymphocytes that is responsible for recognizing antigens bound to major histocompatibility complex molecules...
, only cells that have successfully rearranged the beta chain to produce a functional pre-TCR are allowed to develop beyond the DN3 stage. Cells that fail to produce a functional pre-TCR are eliminated by apoptosis
Apoptosis
Apoptosis is the process of programmed cell death that may occur in multicellular organisms. Biochemical events lead to characteristic cell changes and death. These changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentation...
. This process is referred to as the beta-selection checkpoint. Successful beta-selection requires that TCRβ is produced, TCRβ is capable of pairing with pre-Tα to generate the pre-TCR, and that the pre-TCR can interact on the cell surface with the TCR signalling proteins.
Following β-selection thymocytes generate CD4+CD8+ double positive cells, which then undergo TCRα rearrangement, resulting in completely assembled TCR.
Positive selection and lineage commitment
Thymocytes which pass "β-selection" express a T cell receptorT cell receptor
The T cell receptor or TCR is a molecule found on the surface of T lymphocytes that is responsible for recognizing antigens bound to major histocompatibility complex molecules...
which is capable of assembling on the surface. However many of these T cell receptor
T cell receptor
The T cell receptor or TCR is a molecule found on the surface of T lymphocytes that is responsible for recognizing antigens bound to major histocompatibility complex molecules...
s will still be non-functional, due to an inability to bind MHC
MHC
-Biology:*Myosin heavy chain - part of the motor protein myosin's quaternary protein structure*Major histocompatibility complex - a highly polymorphic region on chromosome 6 with genes particularly involved in immune functions-Colleges:...
. The next major stage of thymocyte development is positive selection, to keep only those thymocytes which have a T cell receptor capable of binding MHC
MHC
-Biology:*Myosin heavy chain - part of the motor protein myosin's quaternary protein structure*Major histocompatibility complex - a highly polymorphic region on chromosome 6 with genes particularly involved in immune functions-Colleges:...
. The T cell receptor requires CD8 as a coreceptor to bind to MHC
MHC
-Biology:*Myosin heavy chain - part of the motor protein myosin's quaternary protein structure*Major histocompatibility complex - a highly polymorphic region on chromosome 6 with genes particularly involved in immune functions-Colleges:...
class I, and CD4
CD4
CD4 is a glycoprotein expressed on the surface of T helper cells, monocytes, macrophages, and dendritic cells. It was discovered in the late 1970s and was originally known as leu-3 and T4 before being named CD4 in 1984...
as a coreceptor to bind MHC
MHC
-Biology:*Myosin heavy chain - part of the motor protein myosin's quaternary protein structure*Major histocompatibility complex - a highly polymorphic region on chromosome 6 with genes particularly involved in immune functions-Colleges:...
class II. At this stage thymocytes upregulate both CD4
CD4
CD4 is a glycoprotein expressed on the surface of T helper cells, monocytes, macrophages, and dendritic cells. It was discovered in the late 1970s and was originally known as leu-3 and T4 before being named CD4 in 1984...
and CD8
CD8
CD8 is a transmembrane glycoprotein that serves as a co-receptor for the T cell receptor . Like the TCR, CD8 binds to a major histocompatibility complex molecule, but is specific for the class I MHC protein. There are two isoforms of the protein, alpha and beta, each encoded by a different gene...
, becoming double positive cells.
Double positive thymocytes that have a T cell receptor capable of binding MHC class I or class II (even with a weak affinity) receive signalling through the T cell receptor. Thymocytes that have a T cell receptor incapable of binding MHC class I or class II undergo apoptosis
Apoptosis
Apoptosis is the process of programmed cell death that may occur in multicellular organisms. Biochemical events lead to characteristic cell changes and death. These changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentation...
. Some thymocytes are able to rescue failed positive selection by receptor editing (rearrangement of the other T cell receptor allele to produce a new T cell receptor).
The double positive thymocytes undergo lineage commitment, maturing into a CD8+ T cell (recognising MHC class I) or a CD4+ T cell (recognising MHC class II). Lineage commitment occurs at the late stage of positive selection and works by downregulation of both CD4 and CD8 (reducing the signal from the T cell receptor) and then upregulation of CD4 only. Thymocytes that start receiving signal again are those that recognise MHC class II, and they become CD4+ T cells. Thymocytes that do not start receiving signal again are those that recognize MHC class I, and they downregulate CD4 and upregulate CD8, to become CD8+ T cells. Both of these thymocytes types are known as single positive thymocytes.
Negative selection
Success in positive selection allows the thymocyte to undergo a number of maturational changes during the transition to a single positive T cell. The single positive T cells upregulate the chemokineChemokine
Chemokines are a family of small cytokines, or proteins secreted by cells. Their name is derived from their ability to induce directed chemotaxis in nearby responsive cells; they are chemotactic cytokines...
receptor CCR7, causing migration from the cortex to the medulla. At this stage the key maturation process involves negative selection, the elimination of autoreactive thymocytes.
The key disadvantage in a gene rearrangement process for T cell receptor
T cell receptor
The T cell receptor or TCR is a molecule found on the surface of T lymphocytes that is responsible for recognizing antigens bound to major histocompatibility complex molecules...
s is that by random chance, some arrangements of gene fragments will create a T cell receptor capable of binding self-peptides presented on MHC class I or MHC class II. If T cells bearing these T cell receptors were to enter the periphery, they would be capable of activating an immune response against self, resulting in autoimmunity
Autoimmunity
Autoimmunity is the failure of an organism to recognize its own constituent parts as self, which allows an immune response against its own cells and tissues. Any disease that results from such an aberrant immune response is termed an autoimmune disease...
. Negative selection is the process evolved to reduce this risk. During negative selection, all thymocytes with a high affinity for binding self peptides presented on MHC class I or class II are induced to upregulate Bim
Bim
Bim is a 1974 Trinidad and Tobago film written by Raoul Pantin and directed by Hugh A. Robertson. It was described by Bruce Paddington as "one of the most important films to be produced in Trinidad and Tobago".-Plot:...
, a protein which drives apoptosis. Cells which do not have a high affinity for self survive negative selection. At this stage, some cells are also selected to become regulatory T cells, usually cells which have an intermediate affinity for self-peptide.
Negative selection can occur at the double positive stage in the cortex. However the repertoire of peptides in the cortex is limited to those expressed by epithelial cells, and double positive cells are poor at undergoing negative selection. Therefore the most important site for negative selection is the medulla, once cells are at the single positive stage. In order to remove thymocytes reactive to peripheral organs, the transcription factor AIRE
Aire
- Rivers :*River Aire, a river in Yorkshire, England*Aire , a river in the Ardennes département, northern France*Aire River , a river in the Canton of Geneva, in Switzerland*Aire River , a river in Australia- Towns :...
drives the expression of multiple peripheral antigens, such as insulin, resulting in deletion of cells specific for those antigens. This allows single positive thymocytes to be exposed to a more complex set of self-antigens than is present in the cortex, and therefore more efficiently deletes those T cells which are autoreactive.
Single positive thymocytes remain in the medulla for 1–2 weeks, surveying self-antigens to test for autoreactivity. During this time they undergo final maturational changes, and then exit the thymus using S1P and CCR7. Upon entry to the peripheral bloodstream, the cells are considered mature T cells, and not thymocytes.
Negative selection is not 100% complete, some autoreactive T cells escape thymic censorship, and are released into the circulation. Additional mechanisms of peripheral tolerance
Peripheral tolerance
Peripheral tolerance is immunological tolerance developed after T and B cells mature and enter the periphery. These include the suppression of autoreactive cells by 'regulatory' T cells and the generation of hyporesponsiveness in lymphocytes which encounter antigen in the absence of the...
active in the periphery exist to silence these cells such as anergy
Anergy
Anergy is a term in immunobiology that describes a lack of reaction by the body's defense mechanisms to foreign substances, and consists of a direct induction of peripheral lymphocyte tolerance. An individual in a state of anergy often indicates that the immune system is unable to mount a normal...
, deletion, and regulatory T cells. If these peripheral tolerance mechanisms also fail, autoimmunity
Autoimmunity
Autoimmunity is the failure of an organism to recognize its own constituent parts as self, which allows an immune response against its own cells and tissues. Any disease that results from such an aberrant immune response is termed an autoimmune disease...
may arise.
Thymus transplantation
Thymus transplantation
Thymus transplantation is a form of organ transplantation where the thymus is moved from one body to another.-Indication:Thymus transplantation can be used to treat infants with DiGeorge syndrome, which results in an absent or hypoplastic thymus, in turn causing problems with the immune system's...
results in that T cells are taught to avoid reacting with donor antigens instead, and may still react with many self-antigens in the body. Autoimmune disease is a frequent complication after thymus transplantation, found in 42% of subjects over 1 year post-transplantation. However, this is partially explained by that the indication itself, that is, complete DiGeorge syndrome
DiGeorge syndrome
22q11.2 deletion syndrome, which has several presentations including DiGeorge syndrome , DiGeorge anomaly, velo-cardio-facial syndrome, Shprintzen syndrome, conotruncal anomaly face syndrome, Strong syndrome, congenital thymic aplasia, and thymic hypoplasia is a syndrome caused by the deletion of a...
(absence of thymus), increases the risk of autoimmune disease.


