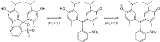
Thymol blue
Encyclopedia
Thymol blue is a brownish-green or reddish-brown crystalline powder that is used as a pH indicator
. It is insoluble in water
but soluble in alcohol
and dilute alkali
solutions. It transitions from red to yellow at pH
1.2–2.8 and from yellow to blue at pH 8.0–9.6.

PH indicator
A pH indicator is a halochromic chemical compound that is added in small amounts to a solution so that the pH of the solution can be determined visually. Hence a pH indicator is a chemical detector for hydronium ions or hydrogen ions in the Arrhenius model. Normally, the indicator causes the...
. It is insoluble in water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
but soluble in alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
and dilute alkali
Alkali
In chemistry, an alkali is a basic, ionic salt of an alkali metal or alkaline earth metal element. Some authors also define an alkali as a base that dissolves in water. A solution of a soluble base has a pH greater than 7. The adjective alkaline is commonly used in English as a synonym for base,...
solutions. It transitions from red to yellow at pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
1.2–2.8 and from yellow to blue at pH 8.0–9.6.


