
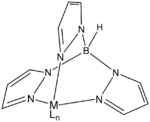
Tp ligand
Encyclopedia
Inorganic chemistry
Inorganic chemistry is the branch of chemistry concerned with the properties and behavior of inorganic compounds. This field covers all chemical compounds except the myriad organic compounds , which are the subjects of organic chemistry...
, the trispyrazolylborate ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
, abbreviated Tp, is a tridentate ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
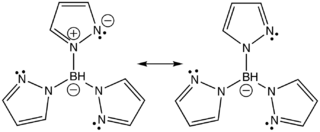
. Trispyrazolylborate refers specifically to the anion [HB(C3N2H3)3]-, but the term trispyrazolylborate refers to derivatives substituted at on the pyrazolyl rings. This family of compounds are sometimes called scorpionate ligand
Scorpionate ligand
The term scorpionate ligand refers to a tridentate ligand which would bind to a metal in a fac manner. The most popular class of scorpionates are the hydrotrisborates or Tp ligands. These were also the first to become popular. These ligands first appeared in journals in 1966 from the then...
.
Tp ligands
As suggested by the resonance structures, the nitrogen centers that are not bonded to boron are basic. These centers bind to three adjacent sites of a metal such that the simple adducts have C3v symmetrySymmetry group
The symmetry group of an object is the group of all isometries under which it is invariant with composition as the operation...
. The facial bonding mode is reminiscent of cyclopentadienyl
Cyclopentadienyl
In organic chemistry, cyclopentadienyl is a cyclic group of atoms with the formula C5H5. Cyclopentadienyl are closely related to cyclopentadiene. Cyclopentadienyl have five carbon atoms bonded together in a pentagonal planar ring, all five of which are bonded to individual hydrogen atoms...
ligands, although the ligand field stabilization energy of Tp- is weaker as indicated by the fact that Fe(Tp)2 is a spin-crossover
Spin crossover
Spin Crossover , sometimes referred to as spin transition or spin equilibrium behavior, is a phenomenon that occurs in some metal complexes wherein the spin state of the complex changes due to external stimuli such as a variation of temperature, pressure, light irradiation or an influence of a...
complex whereas ferrocene
Ferrocene
Ferrocene is an organometallic compound with the formula Fe2. It is the prototypical metallocene, a type of organometallic chemical compound consisting of two cyclopentadienyl rings bound on opposite sides of a central metal atom. Such organometallic compounds are also known as sandwich compounds...
is low-spin.
Pyrazole
Pyrazole refers both to the class of simple aromatic ring organic compounds of the heterocyclic diazole series characterized by a 5-membered ring structure composed of three carbon atoms and two nitrogen atoms in adjacent positions, and to the unsubstituted parent compound...
with potassium borohydride:
- KBH4 + 3 C3H3N2H → K[HB(C3N2H3)3] + 3H2
Intermediates include the monopyrazolylborate ([H3B(C3N2H3)]-) and the bispyrazolylborate ([H2B(C3N2H3)2]-). KTp (m.p. 188-189 °C) is a colourless solid that soluble in polar solvents.
Substituted tris(pyrazolylborate)s
Condensation of 3-substituted pyrazoles with borohydride affords the corresponding substituted Tp derivatives. The substituent forces boron to the less hindered nitrogen center. Thus 3-phenylpyrazole gives HB(C3N2H2Ph)3]-, abbreviated [TpPh]-, wherein the phenyl substituents project away from the metal. Analogously 3-isopropylpyrazole gives HB(C3N2H2iPr)3]-, abbreviated [TpiPr]-. 3,5-Dimethylpyrazole gives the hexamethylated ligand [HB(C3N2H2)3]-, sometimes called Tp*-. Because pyrazolePyrazole
Pyrazole refers both to the class of simple aromatic ring organic compounds of the heterocyclic diazole series characterized by a 5-membered ring structure composed of three carbon atoms and two nitrogen atoms in adjacent positions, and to the unsubstituted parent compound...
s are readily prepared from 1,3-diketones, a large number of substituted Tp complexes are possible. Derivatives are known with perfluorinated, chiral, and functional substituents.
Complexes
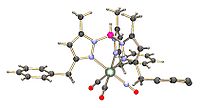
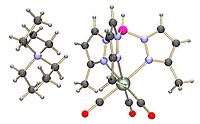
- MnBr(CO)5 + KTp → TpMn(CO)3 + KBr + 2 CO
Electronically related compounds are known, such as CpMn(CO)3
Methylcyclopentadienyl manganese tricarbonyl
Methylcyclopentadienyl manganese tricarbonyl is an organomanganese compound with the formula Mn3. Marketed initially in 1958 as a supplement to the gasoline additive tetraethyl lead to increase the fuel's octane rating, MMT was later used in unleaded gasoline...
and [(9-ane-S3
1,4,7-Trithiacyclononane
1,4,7-Trithiacyclononane, also called 9-ane-S3, is the heterocyclic compound with the formula 3. This cyclic thioether is most often encountered as a tridentate ligand in coordination chemistry....
)Mn(CO)3]+. The labile acetonitrile
Acetonitrile
Acetonitrile is the chemical compound with formula . This colourless liquid is the simplest organic nitrile. It is produced mainly as a byproduct of acrylonitrile manufacture...
complex Mo(CO)3(MeCN)3 reacts with KTp to give the anion [MoTp(CO)3]-, which can be crystallised as its tetraethylammonium
Tetraethylammonium
Tetraethylammonium is a quaternary ammonium cation consisting of four ethyl groups attached to a central nitrogen atom. Like other members of its class, it can be used to alter a compound's solubility by displacing hard acids with this comparatively softer acid...
salt (see figure):
- Mo(CO)3(CH3CN)3 + KTp → K[TpMo(CO)3] + 3 CH3CN
Protonation, allylation, and nitrosylation of this salt gives the corresponding neutral hydride
Hydride
In chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties. In compounds that are regarded as hydrides, hydrogen is bonded to a more electropositive element or group...
, allyl
Allyl
An allyl group is a substituent with the structural formula H2C=CH-CH2R, where R is the connection to the rest of the molecule. It is made up of a methylene , attached to a vinyl group . The name is derived from the Latin word for garlic, Allium sativum. Theodor Wertheim isolated an allyl...
, and nitrosyl (see figure) derivatives.
The inductive effect of substituents on the pyrazolyl groups is illustrated by the values of νCO for TpCF3CuCO (2201 cm-1) vs TpMeCuCO (2137 cm-1).
Although of no practical value, trispyrazolylborate compounds have been applied to a variety of themes. In bioinorganic chemistry
Bioinorganic chemistry
Bioinorganic chemistry is a field that examines the role of metals in biology. Bioinorganic chemistry includes the study of both natural phenomena such as the behavior of metalloproteins as well artificially introduced metals, including those that are non-essential, in medicine and toxicology...
, some of the first crystallizable copper dioxygen complexes were obtained using this ligand platform, including examples of the Cu2(μ-η2,η2-O2) bonding mode. Models for hemerythrin
Hemerythrin
Hemerythrin is an oligomeric protein responsible for oxygen transport in the marine invertebrate phyla of sipunculids, priapulids, brachiopods, and in a single annelid worm, magelona. Recently, hemerythrin was discovered in methanotrophic bacterium Methylococcus capsulatus...
, an enzyme with a diiron active site, and xanthine oxidase
Xanthine oxidase
Xanthine oxidase Xanthine oxidase Xanthine oxidase (XO (sometimes 'XAO'), a form of xanthine oxidoreductase that generates reactive oxygen species. Is an enzyme that catalyzes the oxidation of hypoxanthine to xanthine and can further catalyze the oxidation of xanthine to uric acid...
, a molybdoenzyme, have been examined. In such model complexes, the Tp- simulates the coordination environment provided by three imidazole
Imidazole
Imidazole is an organic compound with the formula C3H4N2. This aromatic heterocyclic is a diazole and is classified as an alkaloid. Imidazole refers to the parent compound, whereas imidazoles are a class of heterocycles with similar ring structure, but varying substituents...
ligands in protein
Protein
Proteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s.
In organometallic chemistry
Organometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
, Tp*Rh(CO)2 and related complexes participate in C-H activation reactions. Derivatives of Grignard reagents, but without the complications of solvation or the Schlenk equilibrium
Schlenk equilibrium
The Schlenk equilibrium is a chemical equilibrium named after its discoverer Wilhelm Schlenk taking place in solutions of Grignard reagents.The process described is an equilibrium between two equivalents of an alkyl or aryl magnesium halide on the left of the equation and on the right side, one...
can be generated, such as TpiBuMgCH3.

