
Triatomic molecule
Encyclopedia
Triatomic molecules are formed by three atoms. Examples include H2O, CO2, HCN etc.
of a triatomic molecule can be determined in specific cases.
In the previous formulas, M is the total mass of the molecule, mA and mB are the masses of the elements A and B, k1 and k2 are the spring constants of the molecule along its axis and perpendicular to it.
 Ozone
Ozone
, O3 is an example of a triatomic molecule with all atoms the same. Triatomic hydrogen
, H3, is unstable and breaks up spontaneously. H3+, the trihydrogen cation is stable by itself and is symmetric.
Molecular vibrations
The vibrational modesMolecular vibration
A molecular vibration occurs when atoms in a molecule are in periodic motion while the molecule as a whole has constant translational and rotational motion...
of a triatomic molecule can be determined in specific cases.
Symmetric linear molecules
A symmetric linear molecule ABA can perform:- Antisymmetric longitudinal vibrations with frequency

- Symmetric longitudinal vibrations with frequency
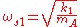
- Symmetric transversal vibrations with frequency
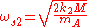
In the previous formulas, M is the total mass of the molecule, mA and mB are the masses of the elements A and B, k1 and k2 are the spring constants of the molecule along its axis and perpendicular to it.
Types


Ozone
Ozone , or trioxygen, is a triatomic molecule, consisting of three oxygen atoms. It is an allotrope of oxygen that is much less stable than the diatomic allotrope...
, O3 is an example of a triatomic molecule with all atoms the same. Triatomic hydrogen
Triatomic hydrogen
Triatomic hydrogen or H3 is an unstable molecule composed of three atoms of hydrogen.The neutral molecule can be formed in a low pressure gas discharge tube.It can break up in the following ways: H_3 \quad \longrightarrow \quad H_3^+ \ + \ e^-...
, H3, is unstable and breaks up spontaneously. H3+, the trihydrogen cation is stable by itself and is symmetric.

