Trimethylamine N-oxide
Encyclopedia
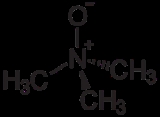
Trimethylamine N-oxide, also known by several other names and acronyms, is the organic compound
with the formula
(CH3)3NO. This colorless solid is usually encountered as the dihydrate
. It is an oxidation product of trimethylamine
and a common metabolite
in animals. It is an osmolyte
found in saltwater fish, sharks and rays, molluscs, and crustaceans. It is a protein stabilizer that may serve to counteract urea, the major osmolyte of sharks, skates and rays. It is also higher in deep-sea fishes and crustaceans, where it may counteract the protein-destabilizing effects of pressure. TMAO decomposes to trimethylamine
(TMA), which is the main odorant that is characteristic of degrading seafood.
Trimethylamine-N-oxide is biosynthesized from trimethylamine
, which is derived from choline
.
is a defect in the production of the enzyme flavin containing monooxygenase 3
(FMO3),, causing incomplete breakdown of trimethylamine from choline
-containing food into trimethylamine oxide. Trimethylamine then builds up and is released in the person's sweat, urine, and breath, giving off a strong fishy odor.
experiments to counteract the unfolding effects of urea
.
In organometallic chemistry
, Me3NO is employed as a decarbonylation agent according to the following stoichiometry:
This reaction is used to decomplex organic ligands from metals, e.g. from (diene)Fe(CO)3.
It is used in certain oxidation reactions, e.g. the conversion of alkyl iodides to the aldehyde
.
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
with the formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
(CH3)3NO. This colorless solid is usually encountered as the dihydrate
Water of crystallization
In crystallography, water of crystallization or water of hydration or crystallization water is water that occurs in crystals. Water of crystallization is necessary for the maintenance of crystalline properties, but capable of being removed by sufficient heat...
. It is an oxidation product of trimethylamine
Trimethylamine
Trimethylamine is an organic compound with the formula N3. This colorless, hygroscopic, and flammable tertiary amine has a strong "fishy" odor in low concentrations and an ammonia-like odor at higher concentrations...
and a common metabolite
Metabolite
Metabolites are the intermediates and products of metabolism. The term metabolite is usually restricted to small molecules. A primary metabolite is directly involved in normal growth, development, and reproduction. Alcohol is an example of a primary metabolite produced in large-scale by industrial...
in animals. It is an osmolyte
Osmolyte
Osmolytes are compounds affecting osmosis. They are soluble in the solution within a cell, or in the surrounding fluid, e.g. as plasma osmolytes. They play a role in maintaining cell volume and fluid balance. For example, when a cell swells due to external osmotic pressure, membrane channels open...
found in saltwater fish, sharks and rays, molluscs, and crustaceans. It is a protein stabilizer that may serve to counteract urea, the major osmolyte of sharks, skates and rays. It is also higher in deep-sea fishes and crustaceans, where it may counteract the protein-destabilizing effects of pressure. TMAO decomposes to trimethylamine
Trimethylamine
Trimethylamine is an organic compound with the formula N3. This colorless, hygroscopic, and flammable tertiary amine has a strong "fishy" odor in low concentrations and an ammonia-like odor at higher concentrations...
(TMA), which is the main odorant that is characteristic of degrading seafood.
Synthesis
Treatment of aqueous trimethylamine with hydrogen peroxide affords the dihydrate (Me = CH3):- H2O2 + Me3N → H2O + Me3NO
Trimethylamine-N-oxide is biosynthesized from trimethylamine
Trimethylamine
Trimethylamine is an organic compound with the formula N3. This colorless, hygroscopic, and flammable tertiary amine has a strong "fishy" odor in low concentrations and an ammonia-like odor at higher concentrations...
, which is derived from choline
Choline
Choline is a water-soluble essential nutrient. It is usually grouped within the B-complex vitamins. Choline generally refers to the various quaternary ammonium salts containing the N,N,N-trimethylethanolammonium cation....
.
Trimethylaminuria
TrimethylaminuriaTrimethylaminuria
Trimethylaminuria , also known as fish odor syndrome or fish malodor syndrome, is a rare metabolic disorder that causes a defect in the normal production of the enzyme Flavin containing monooxygenase 3...
is a defect in the production of the enzyme flavin containing monooxygenase 3
Flavin containing monooxygenase 3
Dimethylaniline monooxygenase [N-oxide-forming] 3 is an enzyme that in humans is encoded by the FMO3 gene.-External Links:* -Further reading:...
(FMO3),, causing incomplete breakdown of trimethylamine from choline
Choline
Choline is a water-soluble essential nutrient. It is usually grouped within the B-complex vitamins. Choline generally refers to the various quaternary ammonium salts containing the N,N,N-trimethylethanolammonium cation....
-containing food into trimethylamine oxide. Trimethylamine then builds up and is released in the person's sweat, urine, and breath, giving off a strong fishy odor.
Laboratory applications
Trimethylamine oxide is used in protein foldingProtein folding
Protein folding is the process by which a protein structure assumes its functional shape or conformation. It is the physical process by which a polypeptide folds into its characteristic and functional three-dimensional structure from random coil....
experiments to counteract the unfolding effects of urea
Urea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
.
In organometallic chemistry
Organometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
, Me3NO is employed as a decarbonylation agent according to the following stoichiometry:
- M(CO)n + Me3NO + L → M(CO)n-1L + Me3N + CO2
This reaction is used to decomplex organic ligands from metals, e.g. from (diene)Fe(CO)3.
It is used in certain oxidation reactions, e.g. the conversion of alkyl iodides to the aldehyde
Aldehyde
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group....
.

