
Trough battery
Encyclopedia
Alessandro Volta
Count Alessandro Giuseppe Antonio Anastasio Gerolamo Umberto Volta was a Lombard physicist known especially for the invention of the battery in 1800.-Early life and works:...
's Voltaic Pile
Voltaic pile
A voltaic pile is a set of individual Galvanic cells placed in series. The voltaic pile, invented by Alessandro Volta in 1800, was the first electric battery...
and was invented by William Cruickshank
William Cruickshank (chemist)
William Cruickshank was a Scottish military surgeon and chemist, and professor of chemistry at the Royal Military Academy, Woolwich.-Career:...
circa 1800.
Disadvantage of the pile
Volta's battery consisted of brineBrine
Brine is water, saturated or nearly saturated with salt .Brine is used to preserve vegetables, fruit, fish, and meat, in a process known as brining . Brine is also commonly used to age Halloumi and Feta cheeses, or for pickling foodstuffs, as a means of preserving them...
-soaked pieces of cloth sandwiched between zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
and copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
discs, piled in a stack. This resulted in electrolyte
Electrolyte
In chemistry, an electrolyte is any substance containing free ions that make the substance electrically conductive. The most typical electrolyte is an ionic solution, but molten electrolytes and solid electrolytes are also possible....
leakage as the weight of the discs squeezed the electrolyte out of the cloth.
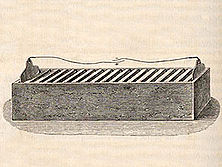
Advantage of the trough
Cruickshank solved this problem by laying the battery on its side in a rectangular box. The inside of this box was lined with shellacShellac
Shellac is a resin secreted by the female lac bug, on trees in the forests of India and Thailand. It is processed and sold as dry flakes , which are dissolved in ethyl alcohol to make liquid shellac, which is used as a brush-on colorant, food glaze and wood finish...
for insulation, and pairs of welded-together zinc and copper plates were laid out in this box, evenly spaced. The spaces between the plates (the troughs) were filled with dilute sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
. So long as the box was not knocked about, there was no risk of electrolyte spillage.

