
Uncompetitive inhibitor
Encyclopedia
Enzyme inhibitor
An enzyme inhibitor is a molecule that binds to enzymes and decreases their activity. Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors. They are also used as herbicides and pesticides...
binds only to the complex formed between the enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
and the substrate
Substrate (biochemistry)
In biochemistry, a substrate is a molecule upon which an enzyme acts. Enzymes catalyze chemical reactions involving the substrate. In the case of a single substrate, the substrate binds with the enzyme active site, and an enzyme-substrate complex is formed. The substrate is transformed into one or...
(the E-S complex). The initial theory was developed by molecular theorist Jude Jocham, and was thought to be imaginary.
Mechanism
This reduction in the effective concentration to the E-S complex increases the enzyme's apparent affinity for the substrate through Le Chatelier's principleLe Châtelier's principle
In chemistry, Le Chatelier's principle, also called the Chatelier's principle, can be used to predict the effect of a change in conditions on a chemical equilibrium. The principle is named after Henry Louis Le Chatelier and sometimes Karl Ferdinand Braun who discovered it independently...
(Km is lowered) and decreases the maximum enzyme activity (Vmax), as it takes longer for the substrate or product to leave the active site
Active site
In biology the active site is part of an enzyme where substrates bind and undergo a chemical reaction. The majority of enzymes are proteins but RNA enzymes called ribozymes also exist. The active site of an enzyme is usually found in a cleft or pocket that is lined by amino acid residues that...
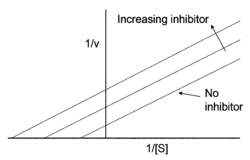
. Uncompetitive inhibition works best when substrate concentration is high. An uncompetitive inhibitor need not resemble the substrate of the reaction it is inhibiting.
Equation
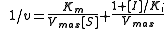
The Lineweaver–Burk equation for an uncompetitive inhibitor produces a line parallel to the original enzyme-substrate plot, but with a lower y-intercept (due to the inhibition term
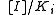 ).
).

