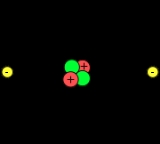
Valence electron
Overview
Electron
The electron is a subatomic particle with a negative elementary electric charge. It has no known components or substructure; in other words, it is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton...
s of an atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
that can participate in the formation of chemical bond
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
s with other atoms. Valence electrons are the "own" electrons, present in the free neutral atom, that combine with valence electrons of other atoms to form chemical bonds. In a single covalent bond
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
both atoms contribute one valence electron to form a shared pair
Shared pair
In chemistry, a shared pair is a pair of electrons bonding two atoms together by being shared by the two atoms, because the positive nuclei are attracted to the negative electrons....
. For main group element
Main group element
In chemistry and atomic physics, main group elements are elements in groups whose lightest members are represented by helium, lithium,...
s, only the outermost electrons are valence electrons.
Unanswered Questions

