Vidarabine
Encyclopedia
Vidarabine or adenine arabinoside is an antiviral drug
which is active against herpes simplex
and varicella zoster virus
es.
Tethya crypta: spongothymidine and spongouridine; which contained an arabinoside sugar rather than a ribose
. These compounds led to the synthesis of a new generation, sugar modified nucleoside analog: vidarabine, and the related compound: cytarabine
. In 2004 these were the only marine related compounds in clinical use.
The drug was first synthesized in 1960 in the Bernard Randall Baker lab at the Stanford Research Institute.
The drug was originally intended as an anti-cancer drug. The anti-viral activity of vidarabine was first described by M. Privat de Garilhe and J. De Rudder in 1964. It was the first nucleoside analog antiviral
to be given systemically and was the first agent to be licensed for the treatment of systematic herpes virus
infection in man. It was University of Alabama at Birmingham
researcher and physician Dr. Richard Whitley in 1976 where the clinical effectiveness of vidarabine was first realized, and vidarabine was used in the treatment of many viral diseases.
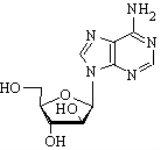
Vidarabine (9-β-D-ribofuranosyladenine) is an analog of adenosine with the D-ribose sugar, replaced with D-arabinose. As you can see from figure 1.1 that it is a stereoisomer of adenosine.
It has a half-life
of 60 minutes, and its solubility is 0.05%, and is able to cross the blood-brain barrier
(BBB) when converted to its active metabolite.
The Mechanism of Action of Vidarabine
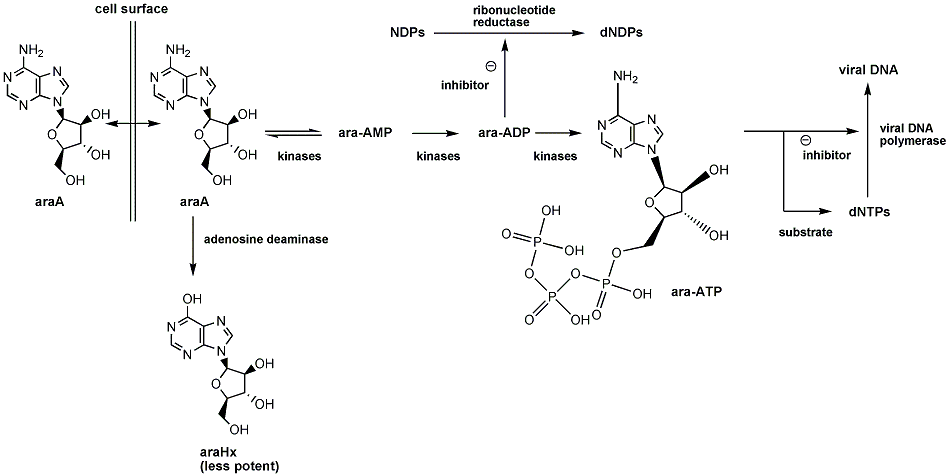
Vidarabine works by interfering with the synthesis of viral DNA. It is a nucleoside analog and therefore has to be phosphorylated to be active. This is a three step process in which vidarabine is sequentially phosphorylated by kinases to the triphosphate ara-ATP. This is the active form of vidarabine and is both an inhibitor and a substrate of viral DNA polymerase.
When used as a substrate for viral DNA polymerase, ara-ATP competitively inhibits dATP leading to the formation of ‘faulty’ DNA. This is where ara-ATP is incorporated into the DNA strand replacing many of the adenosine bases. This results in the prevention of DNA synthesis, as phosphodiester bridges can longer to be built, destabilizing the strand.
Vidarabine triphosphate (ara-ATP) also inhibits RNA polyadenylation; preventing polyadenylation essential for HIV-1 and other retroviruses; and S-adenosylhomocysteine hydrolase, preventing transmethylation reactions.
Uniquely to vidarabine, the diphosphorylated vidarabine (ara-ADP) also has an inhibitory effect. Other nucleoside analogs need to be triphosphorlated to give any antiviral effect, but ara-ADP inhibits the enzyme ribonucleotide reductase. This prevents the reduction of nucleotide diphosphates, causing a reduction of viral replication.
. Viral strains resistant to vidarabine show changes in DNA polymerase
. It is prone to deamination
by adenosine deaminase to a hypoxanthine
. This metabolite still possesses antiviral activity, but is 10-fold less potent than vidarabine. 60% of vidarabine eliminated by the kidney
is excreted as arabinosyle-hypoxanthine in the urine
. Some breakdown of the purine ring may also occur, forming uric acid.
Structural modifications of vidarabine have proven partially effective at blocking deamination, such as replacement of the amine with a methoxy group (ara-M). This results in about a 10-fold greater selectivity against Varicella Zoster Virus
than ara-A, however analog of vidarabine is inactive against other viruses due to it not being able to be phosphorylated.
The use of an inhibitor of adenosine deaminase to increase the half-life of vidarabine has also been tried, and drugs such as dCF and EHNA have been used with a small amount of success.
with a Lewis acid catalyst. This involves the reaction of a base with a sugar and is used to synthesise natural ββ-nucleosides.
The half-life of the active triphosphate metabolite (ara-ATP) is three times longer in HSV-infected cells compared with uninfected cells, however the mechanism of selectivity is not known.
tumour viruses. A 3% ophthalmic ointment Vira-A is used in the treatment of acute keratoconjuctivitis and recurrent superficial keratitis caused by HSV-1 and HSV-2. Vidarabine is also used to treat herpes zoster in AIDS
patients, reducing lesions formation and the duration of viral shedding.
Many of the previous uses of vidarabine have been superseded by acyclovir, due to the hospitalisation required for intra venous dosing, and acyclovir has a higher selectivity, lower inhibitory concentration and higher potency.
Toxic side effects are rare, but have been reported with high concentrations of vidarabine, such as nausea, vomiting, leukopenia
and thrombocytopenia
in patients receiving high intravenous doses daily.
Antiviral drug
Antiviral drugs are a class of medication used specifically for treating viral infections. Like antibiotics for bacteria, specific antivirals are used for specific viruses...
which is active against herpes simplex
Herpes simplex
Herpes simplex is a viral disease caused by both Herpes simplex virus type 1 and type 2 . Infection with the herpes virus is categorized into one of several distinct disorders based on the site of infection. Oral herpes, the visible symptoms of which are colloquially called cold sores or fever...
and varicella zoster virus
Varicella zoster virus
Varicella zoster virus is one of eight herpes viruses known to infect humans . It commonly causes chicken-pox in children and Herpes zoster in adults and rarely in children.-Nomenclature:...
es.
How the drug was discovered
In the 1950’s two nucleosides were isolated from the Caribbean spongeSea sponge
Sponges are animals of the phylum Porifera . Their bodies consist of jelly-like mesohyl sandwiched between two thin layers of cells. While all animals have unspecialized cells that can transform into specialized cells, sponges are unique in having some specialized cells, but can also have...
Tethya crypta: spongothymidine and spongouridine; which contained an arabinoside sugar rather than a ribose
Ribose
Ribose is an organic compound with the formula C5H10O5; specifically, a monosaccharide with linear form H––4–H, which has all the hydroxyl groups on the same side in the Fischer projection....
. These compounds led to the synthesis of a new generation, sugar modified nucleoside analog: vidarabine, and the related compound: cytarabine
Cytarabine
Cytarabine, or cytosine arabinoside, is a chemotherapy agent used mainly in the treatment of cancers of white blood cells such as acute myeloid leukemia and non-Hodgkin lymphoma. It is also known as Ara-C...
. In 2004 these were the only marine related compounds in clinical use.
The drug was first synthesized in 1960 in the Bernard Randall Baker lab at the Stanford Research Institute.
The drug was originally intended as an anti-cancer drug. The anti-viral activity of vidarabine was first described by M. Privat de Garilhe and J. De Rudder in 1964. It was the first nucleoside analog antiviral
Antiviral drug
Antiviral drugs are a class of medication used specifically for treating viral infections. Like antibiotics for bacteria, specific antivirals are used for specific viruses...
to be given systemically and was the first agent to be licensed for the treatment of systematic herpes virus
Herpes virus
In colloquial use, herpes virus refers to the herpes simplex virus, particularly when transmitted sexually.In scientific use, herpesvirus refers to a virus of the taxonomic family herpesviridae....
infection in man. It was University of Alabama at Birmingham
University of Alabama at Birmingham
The University of Alabama at Birmingham is a public university in Birmingham in the U.S. state of Alabama. Developing from an extension center established in 1936, the institution became an autonomous institution in 1969 and is today one of three institutions in the University of Alabama System...
researcher and physician Dr. Richard Whitley in 1976 where the clinical effectiveness of vidarabine was first realized, and vidarabine was used in the treatment of many viral diseases.
Vidarabine (9-β-D-ribofuranosyladenine) is an analog of adenosine with the D-ribose sugar, replaced with D-arabinose. As you can see from figure 1.1 that it is a stereoisomer of adenosine.
It has a half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of 60 minutes, and its solubility is 0.05%, and is able to cross the blood-brain barrier
Blood-brain barrier
The blood–brain barrier is a separation of circulating blood and the brain extracellular fluid in the central nervous system . It occurs along all capillaries and consists of tight junctions around the capillaries that do not exist in normal circulation. Endothelial cells restrict the diffusion...
(BBB) when converted to its active metabolite.
Mode of Action

The Mechanism of Action of Vidarabine
Vidarabine works by interfering with the synthesis of viral DNA. It is a nucleoside analog and therefore has to be phosphorylated to be active. This is a three step process in which vidarabine is sequentially phosphorylated by kinases to the triphosphate ara-ATP. This is the active form of vidarabine and is both an inhibitor and a substrate of viral DNA polymerase.
When used as a substrate for viral DNA polymerase, ara-ATP competitively inhibits dATP leading to the formation of ‘faulty’ DNA. This is where ara-ATP is incorporated into the DNA strand replacing many of the adenosine bases. This results in the prevention of DNA synthesis, as phosphodiester bridges can longer to be built, destabilizing the strand.
Vidarabine triphosphate (ara-ATP) also inhibits RNA polyadenylation; preventing polyadenylation essential for HIV-1 and other retroviruses; and S-adenosylhomocysteine hydrolase, preventing transmethylation reactions.
Uniquely to vidarabine, the diphosphorylated vidarabine (ara-ADP) also has an inhibitory effect. Other nucleoside analogs need to be triphosphorlated to give any antiviral effect, but ara-ADP inhibits the enzyme ribonucleotide reductase. This prevents the reduction of nucleotide diphosphates, causing a reduction of viral replication.
Mode of Resistance
Vidarabine is more toxic and less metabolically stable than many of the other current antivirals such as acyclovir and ganciclovirGanciclovir
Ganciclovir INN is an antiviral medication used to treat or prevent cytomegalovirus infections.Ganciclovir sodium is marketed under the trade names Cytovene and Cymevene . Ganciclovir for ocular use is marketed under the trade name Vitrasert...
. Viral strains resistant to vidarabine show changes in DNA polymerase
DNA polymerase
A DNA polymerase is an enzyme that helps catalyze in the polymerization of deoxyribonucleotides into a DNA strand. DNA polymerases are best known for their feedback role in DNA replication, in which the polymerase "reads" an intact DNA strand as a template and uses it to synthesize the new strand....
. It is prone to deamination
Deamination
Deamination is the removal of an amine group from a molecule. Enzymes which catalyse this reaction are called deaminases.In the human body, deamination takes place primarily in the liver, however glutamate is also deaminated in the kidneys. Deamination is the process by which amino acids are...
by adenosine deaminase to a hypoxanthine
Hypoxanthine
Hypoxanthine is a naturally occurring purine derivative. It is occasionally found as a constituent of nucleic acids where it is present in the anticodon of tRNA in the form of its nucleoside inosine. It has a tautomer known as 6-Hydroxypurine. Hypoxanthine is a necessary additive in certain cell,...
. This metabolite still possesses antiviral activity, but is 10-fold less potent than vidarabine. 60% of vidarabine eliminated by the kidney
Kidney
The kidneys, organs with several functions, serve essential regulatory roles in most animals, including vertebrates and some invertebrates. They are essential in the urinary system and also serve homeostatic functions such as the regulation of electrolytes, maintenance of acid–base balance, and...
is excreted as arabinosyle-hypoxanthine in the urine
Urine
Urine is a typically sterile liquid by-product of the body that is secreted by the kidneys through a process called urination and excreted through the urethra. Cellular metabolism generates numerous by-products, many rich in nitrogen, that require elimination from the bloodstream...
. Some breakdown of the purine ring may also occur, forming uric acid.
Structural modifications of vidarabine have proven partially effective at blocking deamination, such as replacement of the amine with a methoxy group (ara-M). This results in about a 10-fold greater selectivity against Varicella Zoster Virus
Varicella zoster virus
Varicella zoster virus is one of eight herpes viruses known to infect humans . It commonly causes chicken-pox in children and Herpes zoster in adults and rarely in children.-Nomenclature:...
than ara-A, however analog of vidarabine is inactive against other viruses due to it not being able to be phosphorylated.
The use of an inhibitor of adenosine deaminase to increase the half-life of vidarabine has also been tried, and drugs such as dCF and EHNA have been used with a small amount of success.
Synthesis/preparation/isolation
Vidarabine has been synthesised using E. coli bacterial cells, and by using Vorbrüggen glycosylationGlycosylation
Glycosylation is the reaction in which a carbohydrate, i.e. a glycosyl donor, is attached to a hydroxyl or other functional group of another molecule . In biology glycosylation refers to the enzymatic process that attaches glycans to proteins, lipids, or other organic molecules...
with a Lewis acid catalyst. This involves the reaction of a base with a sugar and is used to synthesise natural ββ-nucleosides.
Selectivity
Vidarabine is less susceptible to the development of drug resistant strains than other antivirals such as IDU, and has been used successfully in the treatment of IDU resistant viral strains.The half-life of the active triphosphate metabolite (ara-ATP) is three times longer in HSV-infected cells compared with uninfected cells, however the mechanism of selectivity is not known.
Current clinical Indication
Vidarabine is an antiviral, active against herpes viruses, poxviruses, rhabdoviruses, hepadnaviruses and some RNARNA
Ribonucleic acid , or RNA, is one of the three major macromolecules that are essential for all known forms of life....
tumour viruses. A 3% ophthalmic ointment Vira-A is used in the treatment of acute keratoconjuctivitis and recurrent superficial keratitis caused by HSV-1 and HSV-2. Vidarabine is also used to treat herpes zoster in AIDS
AIDS
Acquired immune deficiency syndrome or acquired immunodeficiency syndrome is a disease of the human immune system caused by the human immunodeficiency virus...
patients, reducing lesions formation and the duration of viral shedding.
Many of the previous uses of vidarabine have been superseded by acyclovir, due to the hospitalisation required for intra venous dosing, and acyclovir has a higher selectivity, lower inhibitory concentration and higher potency.
Toxic side effects are rare, but have been reported with high concentrations of vidarabine, such as nausea, vomiting, leukopenia
Leukopenia
Leukopenia is a decrease in the number of white blood cells found in the blood, which places individuals at increased risk of infection....
and thrombocytopenia
Thrombocytopenia
Thrombocytopenia is a relative decrease of platelets in blood.A normal human platelet count ranges from 150,000 to 450,000 platelets per microliter of blood. These limits are determined by the 2.5th lower and upper percentile, so values outside this range do not necessarily indicate disease...
in patients receiving high intravenous doses daily.

