Wieland-Miescher ketone
Encyclopedia
The Wieland–Miescher ketone is a racemic bicyclic diketone
(enedione) and is a versatile synthon which has so far been employed in the total synthesis of more than 50 natural products, predominantly sesquiterpenoids, diterpene
s and steroid
s possessing possible biological properties including anticancer, antimicrobial, antiviral, antineurodegenerative and immunomodulatory activities. The reaction is named after two chemists from Ciba Geigy, Karl Miescher and Peter Wieland (not to be confused with Heinrich Otto Wieland
). Examples of syntheses performed using the optically active enantiomer of this diketone as a starting material are that of ancistrofuran and the Danishefsky total synthesis of Taxol
.
Most advances in total synthesis methods starting from Wieland–Miescher ketone were fueled by the search for alternative methods for the industrial synthesis of contraceptive and other medicinally relevant steroids, an area of research that flourished in the 1960s and 1970s. Wieland–Miescher ketone contains the AB-ring structure of steroids and is for this reason an attractive starting material for the steroid skeleton, an approach used in one successful synthesis of adrenosterone
.
The original Wieland–Miescher ketone is racemic and prepared in a Robinson annulation
of 2-methyl-1,3-cyclohexanedione and methyl vinyl ketone
. The intermediate alcohol
is not isolated. The required 2-methyl-1,3-cyclohexanedione can be prepared from resorcinol
by hydrogenation
over Raney nickel
to dihydroresorcinol as the enolate followed by alkylation
with methyl iodide.
An enantioselective synthesis employs L-proline as an organocatalyst:
This reaction appeared in 1971 in the patent literature by Z. G. Hajos and D. R. Parrish. In this patent, the isolation and characterization of the above pictured optically active intermediate bicyclic ketol (in parenthesis) has also been described, because they worked at ambient temperature in anhydrous dimethylformamide
(DMF) solvent. Working in DMSO solvent does not allow isolation of the bicyclic ketol intermediate, it leads directly to the optically active bicyclic dione. The reaction is called the Hajos-Parrish reaction or the Hajos-Parrish-Eder-Sauer-Wiechert reaction
..
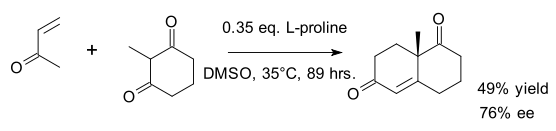
This reaction has also been performed in a one-step procedure, leading to 49% yield
and 76% Enantiomeric excess (ee)
:
Other proline-based catalysts have been investigated
Diketone
A diketone is a molecule containing two ketone groups. The simpliest diketone is diacetyl, also known as 2,3-butanedione. Diacetyl, acetylacetone, and hexane-2,5-dione are examples of 1,2-, 1,3-, and 1,4-diketones, respectively...
(enedione) and is a versatile synthon which has so far been employed in the total synthesis of more than 50 natural products, predominantly sesquiterpenoids, diterpene
Diterpene
Diterpenes are a type of terpenes composed of four isoprene units. They derive from geranylgeranyl pyrophosphate. Diterpenes form the basis for biologically important compounds such as retinol, retinal, and phytol...
s and steroid
Steroid
A steroid is a type of organic compound that contains a characteristic arrangement of four cycloalkane rings that are joined to each other. Examples of steroids include the dietary fat cholesterol, the sex hormones estradiol and testosterone, and the anti-inflammatory drug dexamethasone.The core...
s possessing possible biological properties including anticancer, antimicrobial, antiviral, antineurodegenerative and immunomodulatory activities. The reaction is named after two chemists from Ciba Geigy, Karl Miescher and Peter Wieland (not to be confused with Heinrich Otto Wieland
Heinrich Otto Wieland
Heinrich Otto Wieland was a German chemist. He won the 1927 Nobel Prize in Chemistry for his research into the bile acids. In 1901 Wieland received his doctorate at the University of Munich while studying under Johannes Thiele...
). Examples of syntheses performed using the optically active enantiomer of this diketone as a starting material are that of ancistrofuran and the Danishefsky total synthesis of Taxol
Danishefsky Taxol total synthesis
The Danishefsky Taxol total synthesis in organic chemistry is an important third Taxol synthesis published by the group of Samuel Danishefsky in 1996...
.
Most advances in total synthesis methods starting from Wieland–Miescher ketone were fueled by the search for alternative methods for the industrial synthesis of contraceptive and other medicinally relevant steroids, an area of research that flourished in the 1960s and 1970s. Wieland–Miescher ketone contains the AB-ring structure of steroids and is for this reason an attractive starting material for the steroid skeleton, an approach used in one successful synthesis of adrenosterone
Adrenosterone
Adrenosterone is a steroid hormone with weak androgenic effect. It was first isolated in 1936 from the adrenal cortex by Tadeus Reichstein at the Pharmaceutical Institute in the University of Basel. Originally, adrenosterone was called Reichstein's substance G...
.
The original Wieland–Miescher ketone is racemic and prepared in a Robinson annulation
Robinson annulation
The Robinson annulation is an organic reaction used to create a six-member ring α,β-unsaturated cyclic ketone, using a ketone and methyl vinyl ketone...
of 2-methyl-1,3-cyclohexanedione and methyl vinyl ketone
Methyl vinyl ketone
Methyl vinyl ketone is a reactive organic compound classified as an enone. It is a colorless, flammable, highly toxic liquid with a pungent odor...
. The intermediate alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
is not isolated. The required 2-methyl-1,3-cyclohexanedione can be prepared from resorcinol
Resorcinol
Resorcinol is a dihydroxy benzene. It is the 1,3-isomer of benzenediol with the formula C6H42.-Nomenclature:Benzene-1,3-diol is the name recommended by the International Union of Pure and Applied Chemistry in its 1993 Recommendations for the Nomenclature of Organic Chemistry.-Production:It is...
by hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
over Raney nickel
Raney nickel
Raney nickel is a solid catalyst composed of fine grains of a nickel-aluminium alloy, used in many industrial processes. It was developed in 1926 by American]] engineer Murray Raney as an alternative catalyst for the hydrogenation of vegetable oils in industrial processes...
to dihydroresorcinol as the enolate followed by alkylation
Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion or a carbene . Alkylating agents are widely used in chemistry because the alkyl group is probably the most common group encountered in...
with methyl iodide.
An enantioselective synthesis employs L-proline as an organocatalyst:
This reaction appeared in 1971 in the patent literature by Z. G. Hajos and D. R. Parrish. In this patent, the isolation and characterization of the above pictured optically active intermediate bicyclic ketol (in parenthesis) has also been described, because they worked at ambient temperature in anhydrous dimethylformamide
Dimethylformamide
Dimethylformamide is an organic compound with the formula 2NCH. Commonly abbreviated as DMF , this colourless liquid is miscible with water and the majority of organic liquids. DMF is a common solvent for chemical reactions...
(DMF) solvent. Working in DMSO solvent does not allow isolation of the bicyclic ketol intermediate, it leads directly to the optically active bicyclic dione. The reaction is called the Hajos-Parrish reaction or the Hajos-Parrish-Eder-Sauer-Wiechert reaction
Hajos-Parrish-Eder-Sauer-Wiechert reaction
The Hajos–Parrish–Eder–Sauer–Wiechert reaction in organic chemistry is a proline catalysed asymmetric Aldol reaction. The reaction is named after its principal investigators from Hoffmann-La Roche and Schering AG...
..
This reaction has also been performed in a one-step procedure, leading to 49% yield
Yield (chemistry)
In chemistry, yield, also referred to as chemical yield and reaction yield, is the amount of product obtained in a chemical reaction. The absolute yield can be given as the weight in grams or in moles...
and 76% Enantiomeric excess (ee)
Enantiomeric excess
The enantiomeric excess of a substance is a measure of how pure it is. In this case, the impurity is the undesired enantiomer .-Definition:...
:
Other proline-based catalysts have been investigated