Ytterbium chloride is an inorganic chemical compound.
History
YtterbiumYtterbium is a chemical element with the symbol Yb and atomic number 70. A soft silvery metallic element, ytterbium is a rare earth element of the lanthanide series and is found in the minerals gadolinite, monazite, and xenotime. The element is sometimes associated with yttrium or other related...
, a
LanthanideThe lanthanide or lanthanoid series comprises the fifteen metallic chemical elements with atomic numbers 57 through 71, from lanthanum through lutetium...
series
elementA chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
, was discovered in 1878 by
Marignac who named the element after a town (
YtterbyYtterby is a village on the Swedish island of Resarö, in Vaxholm Municipality in the Stockholm archipelago.The name of the village means "outer village", implying that its location is its most noteworthy feature....
) in
SwedenSweden , officially the Kingdom of Sweden , is a Nordic country on the Scandinavian Peninsula in Northern Europe. Sweden borders with Norway and Finland and is connected to Denmark by a bridge-tunnel across the Öresund....
. The first synthesis of YbCl
3 in the literature was that of Hoogschagen, in 1946. YbCl
3 is now a commercially available source of Yb
3+ ions and therefore of significant chemical interest.
Chemical Properties
The valence electron configuration of Yb
+3 (from YbCl
3) is 4
f135
>s
25
p6, which has crucial implications in chemical behavior of Yb
+3. Also, the size of Yb
+3 governs it’s
catalytic behavior and biological applications. For example, while both
Ce
+3 and Yb
+3 have a single unpaired
f electron, Ce
+3 is
much larger than Yb
+3 because Lanthanides become much smaller with
increasing effective nuclear charge since
f
electrons are not well shielded, compared to
d electrons. This behavior is known as the Lanthanide
contraction. The small size of Yb
+3 produces fast catalytic behavior
and an atomic radius (0.99 Å) comparable to many important biological ions.
The thermodynamic properties tabulated herein were difficult to obtain since many of the measurements were made in the gas phase where YbCl
3
can exist as ([YbCl
6]
-3) or Yb
2Cl
6. The Yb
2Cl
6 species was detected by electron impact
(EI) mass spectrometry as (Yb
2Cl
5+). Additional
complications in obtaining experimental data arise from the myriad of low-lying
f-
d and
f-
f electronic transitions. Despite these issues, the
thermodynamic properties of YbCl
3 have been obtained and the C
3V
symmetryMolecular symmetry in chemistry describes the symmetry present in molecules and the classification of molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can predict or explain many of a molecule's chemical properties, such as its dipole moment...
group has been assigned based upon the four active infrared
vibrations.
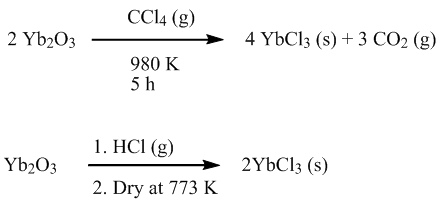
Preparation
YbCl
3 is prepared from
Yb2O3Ytterbium oxide is the chemical compound with the formula Yb2O3. It is one of the more commonly encountered compounds of ytterbium. It has the "rare-earth C-type sesquioxide" structure which is related to the fluorite structure with one quarter of the anions removed, leading to ytterbium atoms in...
with either
carbon tetrachlorideCarbon tetrachloride, also known by many other names is the organic compound with the formula CCl4. It was formerly widely used in fire extinguishers, as a precursor to refrigerants, and as a cleaning agent...
, or hot
hydrochloric acidHydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
.
In practice there are better ways to prepare YbCl
3 for lab use. The aqueous HCl/
ammonium chlorideAmmonium chloride NH4Cl is an inorganic compound with the formula NH4Cl. It is a white crystalline salt that is highly soluble in water. Solutions of ammonium chloride are mildly acidic. Sal ammoniac is a name of natural, mineralogical form of ammonium chloride...
route is unsophisticated but very effective. Alternatively hydrated YbCl
3 may be dehydrated using a variety of reagents, particularly
trimethylsilyl chlorideTrimethylsilyl chloride, also known as chlorotrimethylsilane is a silyl halide, with a variety of different uses in chemistry. It has the formula 3SiCl, and under standard conditions it is a colourless liquid, which is stable in the absence of water...
. Other methods have been published including reacting the finely powdered metal with mercuric chloride at high temperature in a sealed tube. A variety of routes to solvated YbCl
3 have been reported including reaction of the metal with various
halocarbonHalocarbon compounds are chemicals in which one or more carbon atoms are linked by covalent bonds with one or more halogen atoms resulting in the formation of organofluorine compounds, organochlorine compounds, organobromine compounds, and organoiodine compounds...
s in the present of a donor solvent such as
THFTetrahydrofuran is a colorless, water-miscible organic liquid with low viscosity at standard temperature and pressure. This heterocyclic compound has the chemical formula 4O. As one of the most polar ethers with a wide liquid range, it is a useful solvent. Its main use, however, is as a precursor...
, or dehydration of the hydrated chloride using trimethylsilyl or
thionyl chlorideThionyl chloride is an inorganic compound with the formula SOCl2. It is a reactive chemical reagent used in chlorination reactions. It is a colorless, distillable liquid at room temperature and pressure that decomposes above 140 °C. Thionyl chloride is sometimes confused with sulfuryl...
, again in a solvent such as THF.
Catalysis
YbCl
3, with a single unpaired
f electron, acts as a
Lewis acid]The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ a lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair,...
in
order to fill the 4
f orbital. The Lewis acidic nature of YbCl
3 allows YbCl
3 to coordinate (usually
as [YbCl
2]
+) in
transition stateThe transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
s to catalyze
alkylationAlkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion or a carbene . Alkylating agents are widely used in chemistry because the alkyl group is probably the most common group encountered in...
reactions, such as the
aldol reactionThe aldol reaction is a powerful means of forming carbon–carbon bonds in organic chemistry.Discovered independently by Charles-Adolphe Wurtz and Alexander Porfyrevich Borodin in 1872, the reaction combines two carbonyl compounds to form a new β-hydroxy carbonyl compound...
and the
Pictet-Spengler reactionThe Pictet–Spengler reaction is a chemical reaction in which a β-arylethylamine such as tryptamine undergoes ringclosure after condensation with an aldehyde or ketone. Usually an acidic catalyst is employed and the reaction mixture heated, but some reactive compounds give good yields even at...
.
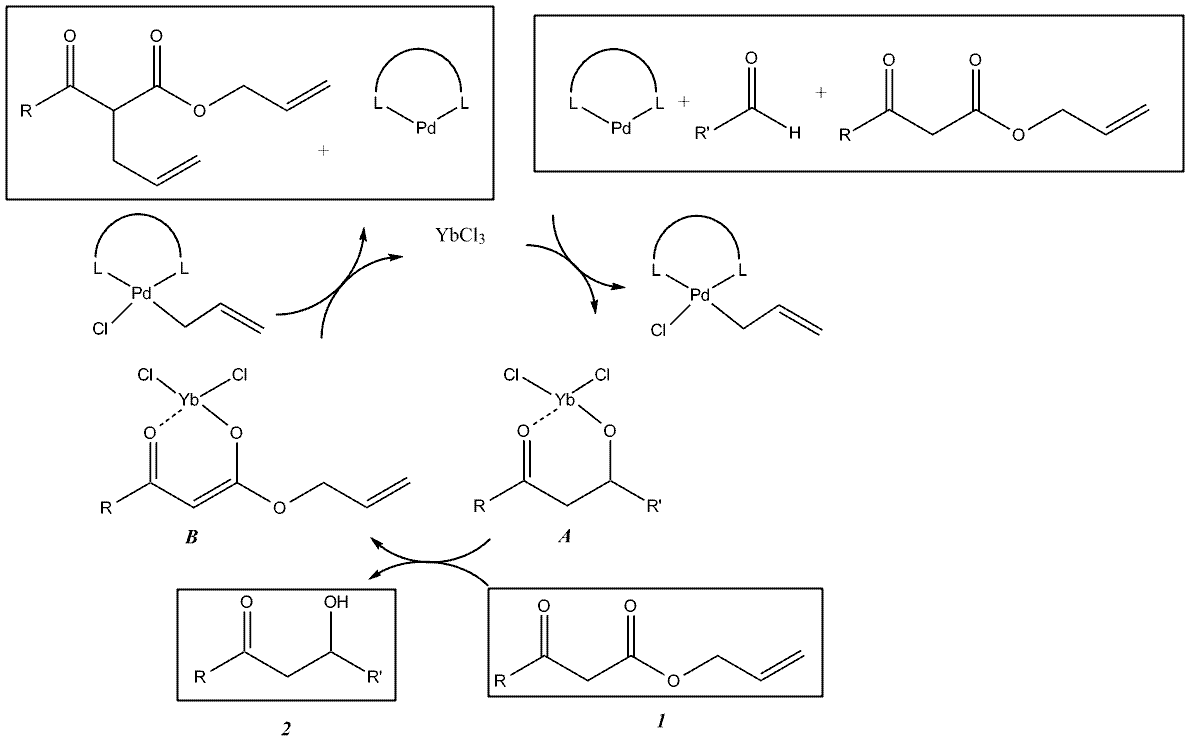
Aldol reaction
The aldol reaction is a versatile reaction in synthetic organic chemistry. YbCl
3 serves a Lewis acid catalyst which aids the
PdPalladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
(0) catalyzed
decarboxylativeDecarboxylation is a chemical reaction that releases carbon dioxide . Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carbonation, the addition of CO2 to...
aldol reaction between a ketone enolate and an aldehyde. Transition states
A and
B show the coordination method of the ytterbium salt as a Lewis acid. For the depicted decarboxylative Aldol reaction with R = tert-butyl and R’ = -(CH
2)
2Ph, the reaction yields show YbCl
3 is an effective Lewis acid catalyst:
Metal salt |
p class=MsoNormal>% yield of2 |
FeCl3Iron chloride, also called ferric chloride, is an industrial scale commodity chemical compound, with the formula FeCl3. The colour of iron chloride crystals depends on the viewing angle: by reflected light the crystals appear dark green, but by transmitted light they appear purple-red...
|
40 |
ZnCl2Zinc chloride is the name of chemical compound with the formula ZnCl2 and its hydrates. Zinc chlorides, of which nine crystalline forms are known, are colorless or white, and are highly soluble in water. ZnCl2 itself is hygroscopic and even deliquescent. Samples should therefore be protected from...
|
68 |
CuCl2Copper chloride is the chemical compound with the formula CuCl2. This is a light brown solid, which slowly absorbs moisture to form a blue-green dihydrate. The copper chlorides are some of the most common copper compounds, after copper sulfate....
|
40 |
LaCl3Lanthanum chloride is the inorganic compound with the formula LaCl3. It is a common salt but is mainly used in research. It is a white solid that is highly soluble in water and alcohols.-Structure:The La3+ centre is 9-coordinate in the trichloride...
|
60 |
YbCl3 |
93 |
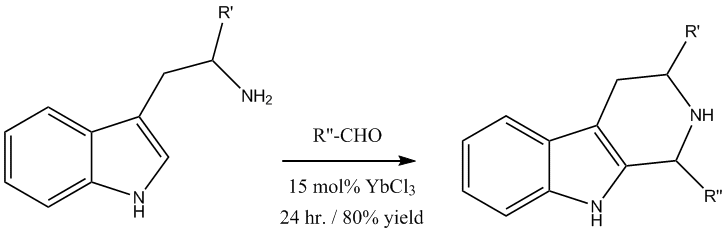
Pictet-Spengler reaction
The Pictet-Spengler reaction produces a valuable tetrahydro-
β-carbolineβ-Carboline is an organic amine that is the prototype of a class of compounds known as β-carbolines.-Pharmacology:...
ring system which can be later used for synthetically prepared indole
alkaloidAlkaloids are a group of naturally occurring chemical compounds that contain mostly basic nitrogen atoms. This group also includes some related compounds with neutral and even weakly acidic properties. Also some synthetic compounds of similar structure are attributed to alkaloids...
s. The Lewis acid catalyzed reaction with YbCl
3 gave excellent yields and reduced reaction times from four days to 24 hours.
Esterification
The small size of Yb
3+ provides fast catalysis, but at the cost of selectivity. For example, the mono-
acetylationAcetylation describes a reaction that introduces an acetyl functional group into a chemical compound...
of
meso-1,2-diols is fastest (2 h) with YbCl
3, but chemoselectivity for the mono-acetylated product is low (50%) compared to CeCl
3 (23
h, 85%).
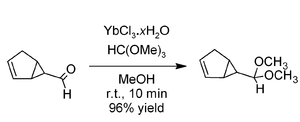
Acetalisation
Ytterbium(III) chloride is a powerful catalyst for the formation of
acetalAn acetal is a molecule with two single-bonded oxygen atoms attached to the same carbon atom.Traditional usages distinguish ketals from acetals...
s using trimethyl orthoformate. In comparison with
cerium(III) chlorideCerium chloride , also known as cerous chloride or cerium trichloride, is a compound of cerium and chlorine. It is a white hygroscopic solid; It rapidly absorbs water on exposure to moist air to form a hydrate which appears to be of variable composition, though the heptahydrate CeCl3·7 H2O is known...
and
erbium(III) chlorideErbium chloride, the erbium salt of hydrochloric acid, is a violet solid used for the preparation of erbium metal.-Properties:It is also found as a pink crystalline hexahydrate, CAS number [10025-75-9]...
, the ytterbium salt was found to be the most effective. Excellent yields are obtained from a variety of aldehydes within a few minutes at room temperature, as in the example above involving an acid-sensitive aldehyde.
Biology
YbCl
3 is a NMR shift reagent that produces different resonances with nuclei in contact with the YbCl
3 versus those nuclei not
in contact with the shift reagent. Generally paramagnetic species, such as a (Lanthanide)
+3 ion, are desirable shift reagents.
Membrane biology has been greatly influenced by YbCl
3, where
39K
+ and
23Na
+ ion movement is critical in establishing
electrochemical gradients. Nerve signaling is a fundamental aspect of life that may be probed with YbCl
3 using NMR
techniques. YbCl
3 may also be used as a calcium ion probe, in a fashion similar
to a sodium ion probe.
YbCl
3 is also used to track digestion in animals.
Certain additives to swine feed, such as probiotics,
may be added to either solid feed or drinking liquids. YbCl
3 travels
with the solid food and therefore helps determine which food phase is ideal to
incorporate the food additive. The YbCl
3 concentration is quantified from ICP (inductively coupled
plasma) mass spectrometry to within 0.0009 μg/mL. YbCl
3 concentration versus time yields the
flow rate of solid particulates in the animal’s digestion. The animal is not
harmed by the YbCl
3 since YbCl
3 is simply excreted in
fecal matter and no change in body weight, organ weight, or hematocrit levels has been observed in mice.
The catalytic nature of YbCl
3 also has an application
in DNA microarrays, or so called DNA “chips”. YbCl
3 led to a 50-80 fold increase in flourescein incorporation into target DNA, which could revolutionize infectious disease
detection (such as a rapid test for tuberculosis).
The source of this article is
wikipedia, the free encyclopedia. The text of this article is licensed under the
GFDL.
_chloride.gif)