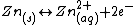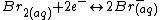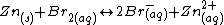Zinc-bromine flow battery
Encyclopedia
The zinc–bromine flow battery
is a type of hybrid flow battery. A solution of zinc bromide
is stored in two tanks. When the battery is charged or discharged the solutions (electrolytes) are pumped through a reactor stack and back into the tanks. One tank is used to store the electrolyte for the positive electrode reactions and the other for the negative. Zinc bromine batteries from different manufacturers have energy densities ranging from 34.4–54 W·h/kg .
The predominantly aqueous electrolyte is composed of zinc bromide salt dissolved in water. During charge, metallic zinc is plated from the electrolyte solution onto the negative electrode surfaces in the cell stacks. Bromide
is converted to bromine
at the positive electrode surface of the cell stack and is immediately stored as safe, chemically complexed organic phase in the electrolyte tank. Each fully recyclable high-density polyethylene (HDPE) cell stack has up to 60 bipolar, plastic electrodes between a pair of anode and cathode end blocks.
The zinc–bromine battery can be regarded as an electroplating
machine. During charging zinc is electroplated onto conductive electrodes, while at the same time bromine is formed. On discharge the reverse process occurs, the metallic zinc plated on the negative electrodes dissolves in the electrolyte and is available to be plated again at the next charge cycle
. It can be left fully discharged indefinitely without damage.
The primary features of the zinc bromine battery are:
Three examples of zinc–bromine flow batteries are ZBB Energy Corporation's Zinc Energy Storage System (ZESS), RedFlow Limited's Zinc Bromine Module (ZBM), and Premium Power's Zinc-Flow Technology.
These battery systems have the potential to provide energy storage
solutions at a lower overall cost than other energy storage systems such as lead-acid, vanadium redox
, sodium–sulfur, lithium-ion and others.
is the electroactive species. Zinc has long been used as the negative electrode of primary cell
s. It is a widely available, relatively inexpensive metal which is electronegative, with a standard reduction potential, E° = −0.76 V vs SHE
. However, it is rather stable in contact with neutral and alkaline aqueous solutions. For this reason it is used today in zinc–carbon and alkaline
primaries.
In the zinc–bromine flow battery the negative electrode reaction is the reversible dissolution/ plating of zinc, according to the following equation.
At the positive electrode bromine
is reversibly reduced
to bromide
, (with a standard reduction potential of +1.087 V vs SHE) according to the following equation.
The overall cell reaction is therefore.
The measured potential difference is around 1.67 V per cell (slightly less than that predicted from the standard reduction potentials).
The two electrode chambers of each cell are divided by a membrane (typically a microporous or ion-exchange variety). This helps to prevent bromine from reaching the positive electrode, where it would react with the zinc, causing the battery to self-discharge. To further reduce the self-discharge, and also to reduce the vapor pressure of bromine, complexing agents are added to the positive electrolyte. These react reversibly with the bromine to form an oily red liquid and reduce the Br2 concentration in the electrolyte.
Flow battery
A flow battery is a form of rechargeable battery in which electrolyte containing one or more dissolved electroactive species flows through an electrochemical cell that converts chemical energy directly to electricity...
is a type of hybrid flow battery. A solution of zinc bromide
Zinc bromide
Zinc bromide is a inorganic compound with the chemical formula ZnBr2. It is a colourless salt that shares many properties with zinc chloride , namely a high solubility in water forming acidic solutions, and solubility in organic solvents...
is stored in two tanks. When the battery is charged or discharged the solutions (electrolytes) are pumped through a reactor stack and back into the tanks. One tank is used to store the electrolyte for the positive electrode reactions and the other for the negative. Zinc bromine batteries from different manufacturers have energy densities ranging from 34.4–54 W·h/kg .
The predominantly aqueous electrolyte is composed of zinc bromide salt dissolved in water. During charge, metallic zinc is plated from the electrolyte solution onto the negative electrode surfaces in the cell stacks. Bromide
Bromide
A bromide is a chemical compound containing bromide ion, that is bromine atom with effective charge of −1. The class name can include ionic compounds such as caesium bromide or covalent compounds such as sulfur dibromide.-Natural occurrence:...
is converted to bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
at the positive electrode surface of the cell stack and is immediately stored as safe, chemically complexed organic phase in the electrolyte tank. Each fully recyclable high-density polyethylene (HDPE) cell stack has up to 60 bipolar, plastic electrodes between a pair of anode and cathode end blocks.
The zinc–bromine battery can be regarded as an electroplating
Electroplating
Electroplating is a plating process in which metal ions in a solution are moved by an electric field to coat an electrode. The process uses electrical current to reduce cations of a desired material from a solution and coat a conductive object with a thin layer of the material, such as a metal...
machine. During charging zinc is electroplated onto conductive electrodes, while at the same time bromine is formed. On discharge the reverse process occurs, the metallic zinc plated on the negative electrodes dissolves in the electrolyte and is available to be plated again at the next charge cycle
Charge cycle
A charge cycle is the process of charging a rechargeable battery and discharging it as required into a load. The term is typically used to specify a battery's expected life, as the number of charge cycles affects life more than the mere passage of time...
. It can be left fully discharged indefinitely without damage.
The primary features of the zinc bromine battery are:
- High energy density relative to lead–acid batteries
- 100% depth of discharge capability on a daily basis
- High cycle life of > 2,000 cycles at 100% depth of discharge, at which point the battery can be serviced to increase cycle life to over 3,500 cycles
- No shelf life limitations as zinc–bromine batteries are non-perishable, unlike lead–acid and lithium-ion batteries, for example.
- Scalable capacities from 10 kW·h (0.036 GJ) to over 500 kW·h (1.8 GJ) systems
- The ability to store energy from any electricity generating source
Three examples of zinc–bromine flow batteries are ZBB Energy Corporation's Zinc Energy Storage System (ZESS), RedFlow Limited's Zinc Bromine Module (ZBM), and Premium Power's Zinc-Flow Technology.
These battery systems have the potential to provide energy storage
Energy storage
Energy storage is accomplished by devices or physical media that store some form of energy to perform some useful operation at a later time. A device that stores energy is sometimes called an accumulator....
solutions at a lower overall cost than other energy storage systems such as lead-acid, vanadium redox
Vanadium redox battery
The vanadium redox battery is a type of rechargeable flow battery that employs vanadium ions in different oxidation states to store chemical potential energy...
, sodium–sulfur, lithium-ion and others.
Electrochemistry
At the negative electrode zincZinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
is the electroactive species. Zinc has long been used as the negative electrode of primary cell
Primary cell
A primary cell is any kind of battery in which the electrochemical reaction is not reversible, rendering the cell non-rechargeable. A common example of a primary cell is the disposable battery. Unlike a secondary cell, the reaction cannot be reversed by running a current into the cell; the chemical...
s. It is a widely available, relatively inexpensive metal which is electronegative, with a standard reduction potential, E° = −0.76 V vs SHE
Standard hydrogen electrode
The standard hydrogen electrode , is a redox electrode which forms the basis of the thermodynamic scale of oxidation-reduction potentials...
. However, it is rather stable in contact with neutral and alkaline aqueous solutions. For this reason it is used today in zinc–carbon and alkaline
Alkaline battery
Alkaline batteries are a type of primary batteries dependent upon the reaction between zinc and manganese dioxide . A rechargeable alkaline battery allows reuse of specially designed cells....
primaries.
In the zinc–bromine flow battery the negative electrode reaction is the reversible dissolution/ plating of zinc, according to the following equation.
At the positive electrode bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
is reversibly reduced
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
to bromide
Bromide
A bromide is a chemical compound containing bromide ion, that is bromine atom with effective charge of −1. The class name can include ionic compounds such as caesium bromide or covalent compounds such as sulfur dibromide.-Natural occurrence:...
, (with a standard reduction potential of +1.087 V vs SHE) according to the following equation.
The overall cell reaction is therefore.
The measured potential difference is around 1.67 V per cell (slightly less than that predicted from the standard reduction potentials).
The two electrode chambers of each cell are divided by a membrane (typically a microporous or ion-exchange variety). This helps to prevent bromine from reaching the positive electrode, where it would react with the zinc, causing the battery to self-discharge. To further reduce the self-discharge, and also to reduce the vapor pressure of bromine, complexing agents are added to the positive electrolyte. These react reversibly with the bromine to form an oily red liquid and reduce the Br2 concentration in the electrolyte.