Zirconium tungstate
Encyclopedia
Zirconium tungstate is a metal oxide
with unusual properties. The phase formed at ambient pressure by reaction of ZrO2 and WO3
is a metastable cubic phase, which has negative thermal expansion
characteristics, namely it shrinks over a wide range of temperatures when heated. In contrast to most other ceramics exhibiting negative CTE (coefficient of thermal expansion), the CTE of ZrW2O8 is isotropic and has a large negative magnitude (average CTE of -7.2x10−6K−1) over a wide range of temperature (-273 °C to 777 °C). A number of other phases
are formed at high pressures.
of zirconium tungstate (ZrW2O8) is perhaps one of the most studied materials to exhibit negative thermal expansion
. It has been shown to contract continuously over a previously unprecedented temperature
range of 0.3 to 1050 K (at higher temperatures the material decomposes). Since the structure is cubic, as described below, the thermal contraction is isotropic - equal in all directions. There is much ongoing research attempting to elucidate why the material exhibits such dramatic negative thermal expansion.
This phase is thermodynamically
unstable at room temperature
with respect to the binary oxides ZrO2 and WO3
, but may be synthesised
by heating stoichiometric quantities of these oxides together and then quenching the material by rapidly cooling it from approximately 900 °C to room temperature.
The structure of cubic zirconium tungstate consists of corner-sharing ZrO6 octahedral and WO4 tetrahedral structural units. Its unusual expansion properties are thought to be due to vibrational modes known as Rigid Unit Modes
(RUMs), which involve the coupled rotation of the polyhedral units that make up the structure, and lead to contraction.
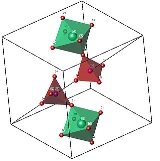
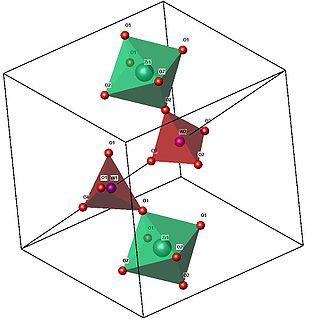 The arrangement of the groups in the structure of cubic ZrW2O8 is analogous to the simple NaCl structure, with ZrO6 octahedra at the Na sites, and W2O8 groups at the Cl sites. The unit cell consists of 44 atoms aligned in a primitive cubic Bravais lattice, with unit cell length 9.15462 Angstrom
The arrangement of the groups in the structure of cubic ZrW2O8 is analogous to the simple NaCl structure, with ZrO6 octahedra at the Na sites, and W2O8 groups at the Cl sites. The unit cell consists of 44 atoms aligned in a primitive cubic Bravais lattice, with unit cell length 9.15462 Angstrom
s.
The ZrO6 octahedra are only slightly distorted from a regular conformation, and all oxygen sites in a given octahedron are related by symmetry. The W2O8 unit is made up of two crystallographically distinct WO4 tetrahedra, which are not formally bonded
to each other. These two types of tetrahedra differ with respect to the W-O bond lengths and angles. The WO4 tetrahedra are distorted from a regular shape since one oxygen is unconstrained (an atom that is bonded only to the central tungsten
(W) atom), and the three other oxygens are each bonded to a zirconium atom (i.e. the corner-sharing of polyhedra).
The structure has P213 space group symmetry
at low temperatures. At higher temperatures, a centre of inversion is introduced by the disordering of the orientation of tungstate groups, and the space group above the phase transition
temperature (~180C) is Pa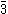 .
.
Octahedra and tetrahedra are linked together by sharing an oxygen atom. In the image, note the corner-touching between octahedra and tetrahedra; these are the location of the shared oxygen
. The vertices of the tetrahedra and octahedra represent the oxygen, which are spread about the central zirconium
and tungsten
. Geometrically, the two shapes can "pivot" around these corner-sharing oxygens, without a distortion of the polyhedra themselves. This pivoting is what is thought to lead to the negative thermal expansion
, as in certain low frequency normal modes this leads to the contracting 'RUMs' mentioned above.
, zirconium tungstate undergoes a series of phase transitions, first to an amorphous phase, and then to a U3O8
-type phase, in which the zirconium and tungsten atoms are disordered.
Oxide
An oxide is a chemical compound that contains at least one oxygen atom in its chemical formula. Metal oxides typically contain an anion of oxygen in the oxidation state of −2....
with unusual properties. The phase formed at ambient pressure by reaction of ZrO2 and WO3
Tungsten oxide
Tungsten has several oxidation states, and therefore oxides:*Tungsten oxide*Tungsten oxide, also known as tungsten dioxide*Tungsten oxide, also known as tungsten trioxide...
is a metastable cubic phase, which has negative thermal expansion
Negative thermal expansion
Negative Thermal Expansion is a physicochemical process in which some materials contract upon heating rather than expanding as most materials do. Materials which undergo this unusual process have a range of potential engineering, photonic, electronic, and structural applications...
characteristics, namely it shrinks over a wide range of temperatures when heated. In contrast to most other ceramics exhibiting negative CTE (coefficient of thermal expansion), the CTE of ZrW2O8 is isotropic and has a large negative magnitude (average CTE of -7.2x10−6K−1) over a wide range of temperature (-273 °C to 777 °C). A number of other phases
Phase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
are formed at high pressures.
Cubic phase
Cubic zirconium tungstate (alpha-ZrW2O8), one of the several known phasesPhase (matter)
In the physical sciences, a phase is a region of space , throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, and chemical composition...
of zirconium tungstate (ZrW2O8) is perhaps one of the most studied materials to exhibit negative thermal expansion
Negative thermal expansion
Negative Thermal Expansion is a physicochemical process in which some materials contract upon heating rather than expanding as most materials do. Materials which undergo this unusual process have a range of potential engineering, photonic, electronic, and structural applications...
. It has been shown to contract continuously over a previously unprecedented temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
range of 0.3 to 1050 K (at higher temperatures the material decomposes). Since the structure is cubic, as described below, the thermal contraction is isotropic - equal in all directions. There is much ongoing research attempting to elucidate why the material exhibits such dramatic negative thermal expansion.
This phase is thermodynamically
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
unstable at room temperature
Room temperature
-Comfort levels:The American Society of Heating, Refrigerating and Air-Conditioning Engineers has listings for suggested temperatures and air flow rates in different types of buildings and different environmental circumstances. For example, a single office in a building has an occupancy ratio per...
with respect to the binary oxides ZrO2 and WO3
Tungsten oxide
Tungsten has several oxidation states, and therefore oxides:*Tungsten oxide*Tungsten oxide, also known as tungsten dioxide*Tungsten oxide, also known as tungsten trioxide...
, but may be synthesised
Chemical synthesis
In chemistry, chemical synthesis is purposeful execution of chemical reactions to get a product, or several products. This happens by physical and chemical manipulations usually involving one or more reactions...
by heating stoichiometric quantities of these oxides together and then quenching the material by rapidly cooling it from approximately 900 °C to room temperature.
The structure of cubic zirconium tungstate consists of corner-sharing ZrO6 octahedral and WO4 tetrahedral structural units. Its unusual expansion properties are thought to be due to vibrational modes known as Rigid Unit Modes
Rigid Unit Modes
Rigid unit modes represent a class of lattice vibrations or phonons that exist in network materials such as quartz, cristobalite or zirconium tungstate. Network materials can be described as three-dimensional networks of polyhedral groups of atoms such as SiO4 tetrahedra or TiO6 octahedra...
(RUMs), which involve the coupled rotation of the polyhedral units that make up the structure, and lead to contraction.
Detailed crystal structure

Ångström
The angstrom or ångström, is a unit of length equal to 1/10,000,000,000 of a meter . Its symbol is the Swedish letter Å....
s.
The ZrO6 octahedra are only slightly distorted from a regular conformation, and all oxygen sites in a given octahedron are related by symmetry. The W2O8 unit is made up of two crystallographically distinct WO4 tetrahedra, which are not formally bonded
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
to each other. These two types of tetrahedra differ with respect to the W-O bond lengths and angles. The WO4 tetrahedra are distorted from a regular shape since one oxygen is unconstrained (an atom that is bonded only to the central tungsten
Tungsten
Tungsten , also known as wolfram , is a chemical element with the chemical symbol W and atomic number 74.A hard, rare metal under standard conditions when uncombined, tungsten is found naturally on Earth only in chemical compounds. It was identified as a new element in 1781, and first isolated as...
(W) atom), and the three other oxygens are each bonded to a zirconium atom (i.e. the corner-sharing of polyhedra).
The structure has P213 space group symmetry
Space group
In mathematics and geometry, a space group is a symmetry group, usually for three dimensions, that divides space into discrete repeatable domains.In three dimensions, there are 219 unique types, or counted as 230 if chiral copies are considered distinct...
at low temperatures. At higher temperatures, a centre of inversion is introduced by the disordering of the orientation of tungstate groups, and the space group above the phase transition
Phase transition
A phase transition is the transformation of a thermodynamic system from one phase or state of matter to another.A phase of a thermodynamic system and the states of matter have uniform physical properties....
temperature (~180C) is Pa
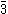 .
.Octahedra and tetrahedra are linked together by sharing an oxygen atom. In the image, note the corner-touching between octahedra and tetrahedra; these are the location of the shared oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
. The vertices of the tetrahedra and octahedra represent the oxygen, which are spread about the central zirconium
Zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name of zirconium is taken from the mineral zircon. Its atomic mass is 91.224. It is a lustrous, grey-white, strong transition metal that resembles titanium...
and tungsten
Tungsten
Tungsten , also known as wolfram , is a chemical element with the chemical symbol W and atomic number 74.A hard, rare metal under standard conditions when uncombined, tungsten is found naturally on Earth only in chemical compounds. It was identified as a new element in 1781, and first isolated as...
. Geometrically, the two shapes can "pivot" around these corner-sharing oxygens, without a distortion of the polyhedra themselves. This pivoting is what is thought to lead to the negative thermal expansion
Negative thermal expansion
Negative Thermal Expansion is a physicochemical process in which some materials contract upon heating rather than expanding as most materials do. Materials which undergo this unusual process have a range of potential engineering, photonic, electronic, and structural applications...
, as in certain low frequency normal modes this leads to the contracting 'RUMs' mentioned above.
High pressure forms
At high pressureHigh pressure
High pressure in science and engineering is studying the effects of high pressure on materials and the design and construction of devices, such as a diamond anvil cell, which can create high pressure...
, zirconium tungstate undergoes a series of phase transitions, first to an amorphous phase, and then to a U3O8
Triuranium octaoxide
Triuranium octoxide is a compound of uranium. It is present as an olive green to black, odorless solid. In spite of its color, it is one of the more popular forms of yellowcake and is shipped between mills and refineries in this form....
-type phase, in which the zirconium and tungsten atoms are disordered.

