1,2-Wittig rearrangement
Encyclopedia
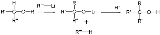
A 1,2-Wittig rearrangement is a categorization of chemical reactions
in organic chemistry
, and consists of a 1,2-rearrangement
of an ether
with an alkyllithium compound. The reaction is named for Nobel Prize
winning chemist Georg Wittig
.
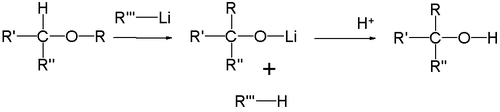 The intermediate product is an alkoxy lithium salt and the final product an alcohol
The intermediate product is an alkoxy lithium salt and the final product an alcohol
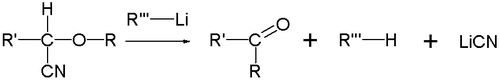
. When R2 is a good leaving group
and electron withdrawing
functional group
such as a cyanide
(CN) group, this group is eliminated and the corresponding ketone
is formed.
centers on the formation of a free radical pair with lithium migrating from the carbon atom to the oxygen atom. The R radical then recombines with the ketyl
.
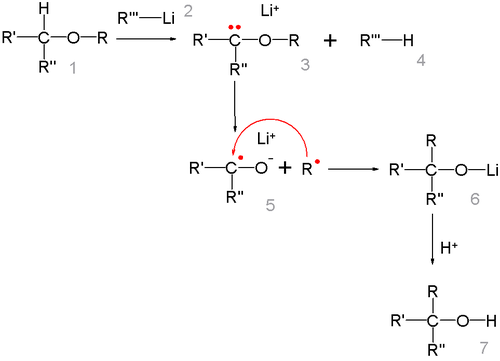 The alkyl group migrates in the order of thermodynamical stability methyl < primary alkyl < secondary alkyl < tertiary alkyl in this is line with the radical mechanism. The radical-ketyl pair is short lived and due to a solvent cage effect
The alkyl group migrates in the order of thermodynamical stability methyl < primary alkyl < secondary alkyl < tertiary alkyl in this is line with the radical mechanism. The radical-ketyl pair is short lived and due to a solvent cage effect
some isomerizations take place with retention of configuration.
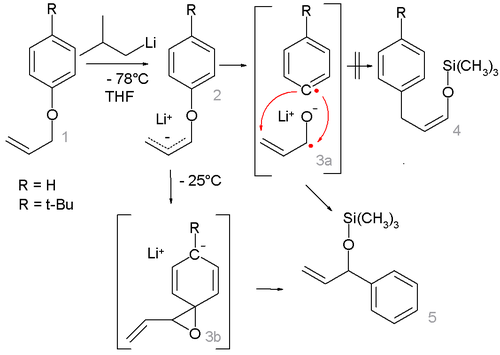
With certain allyl aryl ethers a competing reaction mechanism takes place. The reaction of allyl phenyl ether 1 with sec-butyllithium at -78°C gives the lithiated intermediate 2 which on heating to -25°C only shows the rearranged product 5 but not 4 after trapping the lithium alkoxide with trimethylsilyl chloride
. This result rules out a radical-ketyl intermediate 3a in favor of the Meisenheimer complex
3b. Additional evidence for this mechanism is provided by the finding that with a para tert-butyl substituent the reaction is retarded.
 The reaction is a formal dyotropic reaction
The reaction is a formal dyotropic reaction
.
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
, and consists of a 1,2-rearrangement
1,2-rearrangement
A 1,2-rearrangement or 1,2-migration or 1,2-shift or Whitmore 1,2-shift is an organic reaction where a substituent moves from one atom to another atom in a chemical compound. In a 1,2 shift the movement involves two adjacent atoms but moves over larger distances are possible...
of an ether
Ether
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'. A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether"...
with an alkyllithium compound. The reaction is named for Nobel Prize
Nobel Prize in Chemistry
The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outstanding contributions in chemistry, physics, literature,...
winning chemist Georg Wittig
Georg Wittig
Georg Wittig was a German chemist who reported a method for synthesis of alkenes from aldehydes and ketones using compounds called phosphonium ylides in the Wittig reaction. He shared the Nobel Prize in Chemistry with Herbert C...
.

Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
. When R2 is a good leaving group
Leaving group
In chemistry, a leaving group is a molecular fragment that departs with a pair of electrons in heterolytic bond cleavage. Leaving groups can be anions or neutral molecules. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters, such as para-toluenesulfonate...
and electron withdrawing
Polar effect
The Polar effect or electronic effect in chemistry is the effect exerted by a substituent on modifying electrostatic forces operating on a nearby reaction center...
functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
such as a cyanide
Cyanide
A cyanide is a chemical compound that contains the cyano group, -C≡N, which consists of a carbon atom triple-bonded to a nitrogen atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic....
(CN) group, this group is eliminated and the corresponding ketone
Ketone
In organic chemistry, a ketone is an organic compound with the structure RCR', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group bonded to two other carbon atoms. Many ketones are known and many are of great importance in industry and in biology...
is formed.

Reaction mechanism
The reaction mechanismReaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
centers on the formation of a free radical pair with lithium migrating from the carbon atom to the oxygen atom. The R radical then recombines with the ketyl
Ketyl
A ketyl group in organic chemistry is an anion radical with the general structure C-O. in which an oxygen radical is bonded directly to carbon. This radical is very unstable and appears in chemical reactions as a reactive intermediate...
.

Cage effect (chemistry)
The cage effect in chemistry describes how properties of a molecule are affected by its surroundings.In a solvent a molecule is often more accurately described existing in a cage of solvent molecules, the so-called solvent cage. Reactions occur when a molecule occasionally "jumps out" and meets...
some isomerizations take place with retention of configuration.
With certain allyl aryl ethers a competing reaction mechanism takes place. The reaction of allyl phenyl ether 1 with sec-butyllithium at -78°C gives the lithiated intermediate 2 which on heating to -25°C only shows the rearranged product 5 but not 4 after trapping the lithium alkoxide with trimethylsilyl chloride
Trimethylsilyl chloride
Trimethylsilyl chloride, also known as chlorotrimethylsilane is a silyl halide, with a variety of different uses in chemistry. It has the formula 3SiCl, and under standard conditions it is a colourless liquid, which is stable in the absence of water...
. This result rules out a radical-ketyl intermediate 3a in favor of the Meisenheimer complex
Meisenheimer complex
A Meisenheimer complex or Jackson-Meisenheimer complex in organic chemistry is a 1:1 reaction adduct between an arene carrying electron withdrawing groups and nucleophile...
3b. Additional evidence for this mechanism is provided by the finding that with a para tert-butyl substituent the reaction is retarded.

Dyotropic reaction
A dyotropic Reaction in organic chemistry is a type of organic reaction and more specifically a pericyclic valence isomerization in which two sigma bonds simultaneously migrate intramolecularly...
.
See also
- The 1,3-Wittig rearrangement
- The 2,3-Wittig rearrangement2,3-Wittig rearrangementThe [2,3]-Wittig rearrangement is the transformation of an allylic ether into a homoallylic alcohol via a concerted, pericyclic process. Because the reaction is concerted, it exhibits a high degree of stereocontrol, and can be employed early in a synthetic route to establish stereochemistry...
- The Smiles rearrangementSmiles rearrangementThe Smiles rearrangement is an organic reaction and a rearrangement reaction. It is an intramolecular nucleophilic aromatic substitution of the type:...

