
Organic reaction
Encyclopedia
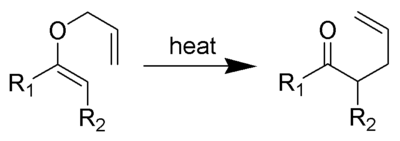
Chemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
s involving organic compound
Organic compound
An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the...
s. The basic organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
reaction types are addition reaction
Addition reaction
An addition reaction, in organic chemistry, is in its simplest terms an organic reaction where two or more molecules combine to form a larger one....
s, elimination reaction
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
s, substitution reaction
Substitution reaction
In a substitution reaction, a functional group in a particular chemical compound is replaced by another group. In organic chemistry, the electrophilic and nucleophilic substitution reactions are of prime importance...
s, pericyclic reaction
Pericyclic reaction
In organic chemistry, a pericyclic reaction is a type of organic reaction wherein the transition state of the molecule has a cyclic geometry, and the reaction progresses in a concerted fashion. Pericyclic reactions are usually rearrangement reactions...
s, rearrangement reaction
Rearrangement reaction
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
s, photochemical reactions
Mechanistic organic photochemistry
Mechanistic organic photochemistry is that aspect of organic photochemistry which seeks to explain the mechanisms of organic photochemical reactions. The absorption of ultraviolet light by organic molecules very often leads to reactions. In the earliest days sunlight was employed while in more...
and redox reactions
Organic redox reaction
Organic reductions or organic oxidations or organic redox reactions are redox reactions that take place with organic compounds. In organic chemistry oxidations and reductions are different from ordinary redox reactions because many reactions carry the name but do not actually involve electron...
. In organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
, organic reactions are used in the construction of new organic molecules. The production of many man-made chemicals such as drugs, plastics, food additives, fabrics depend on organic reactions.
The oldest organic reactions are combustion
Combustion
Combustion or burning is the sequence of exothermic chemical reactions between a fuel and an oxidant accompanied by the production of heat and conversion of chemical species. The release of heat can result in the production of light in the form of either glowing or a flame...
of organic fuels and saponification
Saponification
Saponification is a process that produces soap, usually from fats and lye. In technical terms, saponification involves base hydrolysis of triglycerides, which are esters of fatty acids, to form the sodium salt of a carboxylate. In addition to soap, such traditional saponification processes...
of fats to make soap. Modern organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
starts with the Wöhler synthesis
Wöhler synthesis
rightThe Wöhler synthesis is the conversion of ammonium cyanate into urea. This chemical reaction was discovered in 1828 by Friedrich Wöhler in an attempt to synthesize ammonium cyanate. It is considered the starting point of modern organic chemistry. Although the Wöhler reaction concerns the...
in 1828. In the history of the Nobel Prize in Chemistry
Nobel Prize in Chemistry
The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outstanding contributions in chemistry, physics, literature,...
awards have been given for the invention of specific organic reactions such as the Grignard reaction
Grignard reaction
The Grignard reaction is an organometallic chemical reaction in which alkyl- or aryl-magnesium halides add to a carbonyl group in an aldehyde or ketone. This reaction is an important tool for the formation of carbon–carbon bonds...
in 1912, the Diels-Alder reaction
Diels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
in 1950, the Wittig reaction
Wittig reaction
The Wittig reaction is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide to give an alkene and triphenylphosphine oxide....
in 1979 and olefin metathesis
Olefin metathesis
Olefin metathesis or transalkylidenation is an organic reaction that entails redistribution of alkylene fragments by the scission of carbon - carbon double bonds in olefins . Its advantages include the creation of fewer sideproducts and hazardous wastes. Yves Chauvin, Robert H. Grubbs, and Richard R...
in 2005.
Classifications
Organic chemistry has a strong tradition of naming a specific reaction to its inventor or inventors and a long list of so-called named reactions exists, conservatively estimated at 1000. A very old named reaction is the Claisen rearrangementClaisen rearrangement
The Claisen rearrangement is a powerful carbon-carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen...
(1912) and a recent named reaction is the Bingel reaction
Bingel reaction
The Bingel reaction in fullerene chemistry is a fullerene cyclopropanation reaction to a methanofullerene first discovered by C. Bingel in 1993 with the bromo derivative of diethyl malonate in the presence of a base such as sodium hydride or DBU...
(1993). When the named reaction is difficult to pronounce or very long as in the Corey-House-Posner-Whitesides reaction
Corey-House-Posner-Whitesides reaction
The Corey–Posner, Whitesides–House synthesis is an organic reaction that involves the reaction of a lithium dialkyl cuprate with an alkyl halide to form a new alkane, an organocopper compound and a lithium halide.-Reaction mechanism:This reaction occurs in two steps...
it helps to use the abbreviation as in the CBS reduction
CBS reduction
The Corey-Itsuno Reduction, also known as the Corey-Bakshi-Shibata reduction, is a chemical reaction in which an achiral ketone is enantioselectively reduced to produce the corresponding chiral, non-racemic alcohol...
. The number of reactions hinting at the actual process taking place is much smaller, for example the ene reaction
Ene reaction
The Ene reaction is a chemical reaction between an alkene with an allylic hydrogen and a compound containing a multiple bond , in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the...
or aldol reaction
Aldol reaction
The aldol reaction is a powerful means of forming carbon–carbon bonds in organic chemistry.Discovered independently by Charles-Adolphe Wurtz and Alexander Porfyrevich Borodin in 1872, the reaction combines two carbonyl compounds to form a new β-hydroxy carbonyl compound...
.
Another approach to organic reactions is by type of organic reagent, many of them inorganic
Inorganic compound
Inorganic compounds have traditionally been considered to be of inanimate, non-biological origin. In contrast, organic compounds have an explicit biological origin. However, over the past century, the classification of inorganic vs organic compounds has become less important to scientists,...
, required in a specific transformation. The major types are oxidizing agent
Oxidizing agent
An oxidizing agent can be defined as a substance that removes electrons from another reactant in a redox chemical reaction...
s such as osmium tetroxide, reducing agent
Reducing agent
A reducing agent is the element or compound in a reduction-oxidation reaction that donates an electron to another species; however, since the reducer loses an electron we say it is "oxidized"...
s such as Lithium aluminium hydride
Lithium aluminium hydride
Lithium aluminium hydride, commonly abbreviated to LAH or known as LithAl, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters,...
, bases
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
such as lithium diisopropylamide
Lithium diisopropylamide
Lithium diisopropylamide is the chemical compound with the formula [2CH]2NLi. Generally abbreviated LDA, it is a strong base used in organic chemistry for the deprotonation of weakly acidic compounds. The reagent has been widely accepted because it is soluble in non-polar organic solvents and it...
and acid
Acid
An acid is a substance which reacts with a base. Commonly, acids can be identified as tasting sour, reacting with metals such as calcium, and bases like sodium carbonate. Aqueous acids have a pH of less than 7, where an acid of lower pH is typically stronger, and turn blue litmus paper red...
s such as sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
.
Fundamentals
Factors governing organic reactions are essentially the same as that of any chemical reactionChemical reaction
A chemical reaction is a process that leads to the transformation of one set of chemical substances to another. Chemical reactions can be either spontaneous, requiring no input of energy, or non-spontaneous, typically following the input of some type of energy, such as heat, light or electricity...
. Factors specific to organic reactions are those that determine the stability of reactants and products such as conjugation
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
, hyperconjugation
Hyperconjugation
In organic chemistry, hyperconjugation is the interaction of the electrons in a sigma bond with an adjacent empty non-bonding p-orbital or antibonding π orbital or filled π orbital, to give an extended molecular orbital that increases the stability of the system...
and aromaticity
Aromaticity
In organic chemistry, Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August...
and the presence and stability of reactive intermediate
Reactive intermediate
In chemistry a reactive intermediate is a short-lived, high energy, highly reactive molecule. When generated in a chemical reaction it will quickly convert into a more stable molecule. Only in exceptional cases can these compounds be isolated and stored, e.g. low temperatures, matrix isolation...
s such as free radical
Radical (chemistry)
Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
s, carbocation
Carbocation
A carbocation is an ion with a positively-charged carbon atom. The charged carbon atom in a carbocation is a "sextet", i.e. it has only six electrons in its outer valence shell instead of the eight valence electrons that ensures maximum stability . Therefore carbocations are often reactive,...
s and carbanion
Carbanion
A carbanion is an anion in which carbon has an unshared pair of electrons and bears a negative charge usually with three substituents for a total of eight valence electrons. The carbanion exists in a trigonal pyramidal geometry. Formally a carbanion is the conjugate base of a carbon acid.where B...
s.
An organic compound may consist of many isomer
Isomer
In chemistry, isomers are compounds with the same molecular formula but different structural formulas. Isomers do not necessarily share similar properties, unless they also have the same functional groups. There are many different classes of isomers, like stereoisomers, enantiomers, geometrical...
s. Selectivity in terms of regioselectivity
Regioselectivity
In chemistry, regioselectivity is the preference of one direction of chemical bond making or breaking over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base will abstract from an organic molecule, or where...
, diastereoselectivity and enantioselectivity is therefore an important criterion for many organic reactions. The stereochemistry
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
of pericyclic reaction
Pericyclic reaction
In organic chemistry, a pericyclic reaction is a type of organic reaction wherein the transition state of the molecule has a cyclic geometry, and the reaction progresses in a concerted fashion. Pericyclic reactions are usually rearrangement reactions...
s is governed by the Woodward-Hoffmann rules
Woodward-Hoffmann rules
The Woodward–Hoffmann rules devised by Robert Burns Woodward and Roald Hoffmann are a set of rules in organic chemistry predicting the stereochemistry of pericyclic reactions based on orbital symmetry. These include electrocyclic reactions, cycloadditions , sigmatropic reactions, and group transfer...
and that of many elimination reaction
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
s by the Zaitsev's rule
Zaitsev's rule
In chemistry, Zaitsev's rule, Saytzeff's rule or Saytsev's rule named after Alexander Mikhailovich Zaitsev is a rule that states that if more than one alkene can be formed during dehalogenation by an elimination reaction, the more stable alkene is the major product...
.
Organic reactions are important in the production of pharmaceutical
Medication
A pharmaceutical drug, also referred to as medicine, medication or medicament, can be loosely defined as any chemical substance intended for use in the medical diagnosis, cure, treatment, or prevention of disease.- Classification :...
s. In a 2006 review it was estimated that 20% of chemical conversions involved alkylation
Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion or a carbene . Alkylating agents are widely used in chemistry because the alkyl group is probably the most common group encountered in...
s on nitrogen and oxygen atoms, another 20% involved placement and removal of protective groups, 11% involved formation of new carbon-carbon bond
Carbon-carbon bond
A carbon–carbon bond is a covalent bond between two carbon atoms. The most common form is the single bond: a bond composed of two electrons, one from each of the two atoms. The carbon–carbon single bond is a sigma bond and is said to be formed between one hybridized orbital from each...
and 10% involved functional group interconversions.
Organic reactions by mechanism
There is no limit to the number of possible organic reactions and mechanisms. However, certain general patterns are observed that can be used to describe many common or useful reactions. Each reaction has a stepwise reaction mechanismReaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
that explains how it happens, although this detailed description of steps is not always clear from a list of reactants alone. Organic reactions can be organized into several basic types. Some reactions fit into more than one category. For example, some substitution reactions follow an addition-elimination pathway. This overview isn't intended to include every single organic reaction. Rather, it is intended to cover the basic reactions.
| Reaction type | Subtype | Comment |
|---|---|---|
| Addition reaction Addition reaction An addition reaction, in organic chemistry, is in its simplest terms an organic reaction where two or more molecules combine to form a larger one.... s |
electrophilic addition Electrophilic addition In organic chemistry, an electrophilic addition reaction is an addition reaction where, in a chemical compound, a π bond is broken and two new σ bonds are formed... |
include such reactions as halogenation Halogenation Halogenation is a chemical reaction that incorporates a halogen atom into a molecule in substitution of hydrogen atom. Halogenation takes place in the gas phase. There are four types of halogenation: fluorination, chlorination, bromination, and iodination... , hydrohalogenation Hydrohalogenation A hydrohalogenation reaction is the electrophilic addition of hydrohalic acids like hydrogen chloride or hydrogen bromide to alkenes to yield the corresponding haloalkanes.... and hydration Hydration reaction In organic chemistry, a hydration reaction is a chemical reaction in which a hydroxyl group and a hydrogen cation are added to the two carbon atoms bonded together in the carbon-carbon double bond which makes up an alkene functional group. The reaction usually runs in a strong acidic, aqueous... . |
| nucleophilic addition Nucleophilic addition In organic chemistry, a nucleophilic addition reaction is an addition reaction where in a chemical compound a π bond is removed by the creation of two new covalent bonds by the addition of a nucleophile.... |
||
| radical addition | ||
| Elimination reaction Elimination reaction An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism... |
include processes such as dehydration Dehydration reaction In chemistry and the biological sciences, a dehydration reaction is usually defined as a chemical reaction that involves the loss of water from the reacting molecule. Dehydration reactions are a subset of elimination reactions... and are found to follow an E1, E2 or E1cB E1cB elimination reaction The E1cB elimination reaction is a special type of elimination reaction in organic chemistry. This reaction mechanism explains the formation of alkenes from alkyl halides through a carbanion intermediate given specified reaction conditions and specified substrates. The abbreviation stands for ... reaction mechanism Reaction mechanism In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in... |
|
| Substitution reaction Substitution reaction In a substitution reaction, a functional group in a particular chemical compound is replaced by another group. In organic chemistry, the electrophilic and nucleophilic substitution reactions are of prime importance... s |
nucleophilic aliphatic substitution Nucleophilic substitution In organic and inorganic chemistry, nucleophilic substitution is a fundamental class of reactions in which an electron nucleophile selectively bonds with or attacks the positive or partially positive charge of an atom or a group of atoms called the leaving group; the positive or partially positive... |
with SN1 SN1 reaction The SN1 reaction is a substitution reaction in organic chemistry. "SN" stands for nucleophilic substitution and the "1" represents the fact that the rate-determining step is unimolecular... , SN2 SN2 reaction The SN2 reaction is a type of nucleophilic substitution, where a lone pair from a nucleophile attacks an electron deficient electrophilic center and bonds to it, expelling another group called a leaving group. Thus the incoming group replaces the leaving group in one step... and SNi reaction mechanism Reaction mechanism In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in... s |
| nucleophilic aromatic substitution Nucleophilic aromatic substitution right|300px|Aromatic nucleophilic substitutionA nucleophilic aromatic substitution is a substitution reaction in organic chemistry in which the nucleophile displaces a good leaving group, such as a halide, on an aromatic ring... |
||
| nucleophilic acyl substitution Nucleophilic acyl substitution Nucleophilic acyl substitution describes the substitution reaction involving nucleophiles and acyl compounds. Acyl compounds are carboxylic acid derivatives including esters, amides and acid halides... |
||
| electrophilic substitution Electrophilic substitution Electrophilic substitution reactions are chemical reactions in which an electrophile displaces a group in a compound, typically but not always hydrogen. Electrophilic aromatic substitution is characteristic of aromatic compounds and is an important way of introducing functional groups onto benzene... |
||
| electrophilic aromatic substitution Electrophilic aromatic substitution Electrophilic aromatic substitution EAS is an organic reaction in which an atom, usually hydrogen, appended to an aromatic system is replaced by an electrophile... |
||
| radical substitution Radical substitution In organic chemistry, a radical substitution reaction is a substitution reaction involving free radicals as a reactive intermediate.The reaction always involves at least two steps, and possibly a third.... |
||
| Organic redox reaction Organic redox reaction Organic reductions or organic oxidations or organic redox reactions are redox reactions that take place with organic compounds. In organic chemistry oxidations and reductions are different from ordinary redox reactions because many reactions carry the name but do not actually involve electron... s |
are redox reactions specific to organic compound Organic compound An organic compound is any member of a large class of gaseous, liquid, or solid chemical compounds whose molecules contain carbon. For historical reasons discussed below, a few types of carbon-containing compounds such as carbides, carbonates, simple oxides of carbon, and cyanides, as well as the... s and are very common. |
|
| Rearrangement reaction Rearrangement reaction A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule... s |
1,2-rearrangement 1,2-rearrangement A 1,2-rearrangement or 1,2-migration or 1,2-shift or Whitmore 1,2-shift is an organic reaction where a substituent moves from one atom to another atom in a chemical compound. In a 1,2 shift the movement involves two adjacent atoms but moves over larger distances are possible... s |
|
| pericyclic reactions | ||
| metathesis Olefin metathesis Olefin metathesis or transalkylidenation is an organic reaction that entails redistribution of alkylene fragments by the scission of carbon - carbon double bonds in olefins . Its advantages include the creation of fewer sideproducts and hazardous wastes. Yves Chauvin, Robert H. Grubbs, and Richard R... |
In Condensation reaction
Condensation reaction
A condensation reaction is a chemical reaction in which two molecules or moieties combine to form one single molecule, together with the loss of a small molecule. When this small molecule is water, it is known as a dehydration reaction; other possible small molecules lost are hydrogen chloride,...
s a small molecule, usually water
Water
Water is a chemical substance with the chemical formula H2O. A water molecule contains one oxygen and two hydrogen atoms connected by covalent bonds. Water is a liquid at ambient conditions, but it often co-exists on Earth with its solid state, ice, and gaseous state . Water also exists in a...
, is split off when two reactants combine in a chemical reaction. The opposite reaction, when water is consumed in a reaction, is called hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
. Many Polymerization
Polymerization
In polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains...
reactions are derived from organic reactions. They are divided into addition polymerization
Addition polymerization
Chain growth polymerization is a polymerization technique where unsaturated monomer molecules add on to a growing polymer chain one at a time...
s and step-growth polymerization
Step-growth polymerization
Step-growth polymerization refers to a type of polymerization mechanism in which bi-functional or multifunctional monomers react to form first dimers, then trimers, longer oligomers and eventually long chain polymers. Many naturally occurring and some synthetic polymers are produced by step-growth...
s.
In general the stepwise progression of reaction mechanisms can be represented using arrow pushing
Arrow pushing
Arrow pushing or electron pushing is a technique used to describe the progression of organic chemistry reaction mechanisms. In using arrow pushing, "curved arrows" or "curly arrows" are superimposed over the structural formulae of reactants in a chemical equation to show the reaction mechanism...
techniques in which curved arrows are used to track the movement of electrons as starting materials transition to intermediates and products.
Organic reactions by functional groups
Organic reactions can be categorized based on the type of functional groupFunctional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
involved in the reaction as a reactant and the functional group that is formed as a result of this reaction. For example in the Fries rearrangement
Fries rearrangement
The Fries rearrangement, named for the German chemist Karl Theophil Fries, is a rearrangement reaction of a phenyl ester to a hydroxy aryl ketone by catalysis of Lewis acids.It involves migration of an acyl group of phenyl ester to benzene ring.- Mechanism:...
the reactant is an ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
and the reaction product an alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
.
An overview of functional groups with their preparation and reactivity is presented below:
| Functional group | Preparation | Reactions |
|---|---|---|
| Acid anhydride | preparation | reactions |
| Acyl halides | preparation | reactions |
| Acyloins | preparation | reactions |
| Alcohols | preparation | reactions |
| Aldehydes | preparation | reactions |
| Alkanes | preparation | reactions |
| Alkenes | preparation | reactions |
| Alkyl halides | preparation | reactions |
| Alkyl nitrites | preparation Alkyl nitrites Alkyl nitrites are a group of chemical compounds based upon the molecular structure R-ONO. Formally they are alkyl esters of nitrous acid. They are distinct from nitro compounds .... |
reactions Alkyl nitrites Alkyl nitrites are a group of chemical compounds based upon the molecular structure R-ONO. Formally they are alkyl esters of nitrous acid. They are distinct from nitro compounds .... |
| Alkynes | preparation | reactions |
| Amides | preparation | reactions |
| Amine oxide | preparation | reactions |
| Amines | preparation | reactions |
| Arene compounds | preparation | reactions |
| Azides | preparation | reactions |
| Aziridines | preparation | reactions |
| Carboxylic acids | preparation | reactions |
| Cyclopropanes | preparation | reactions |
| Diazo compounds | preparation | reactions |
| Diols | preparation | reactions |
| Esters | preparation | reactions |
| Ethers | preparation | reactions |
| Epoxide | preparation | reactions |
| Haloketones | preparation | reactions |
| Imines | preparation | reactions |
| Isocyanates | preparation | reactions |
| Ketones | preparation | reactions |
| Lactams | preparation | reactions |
| Nitriles | preparation | reactions |
| Nitro compounds | preparation | reactions |
| Phenols | preparation | reactions |
| Thiols | preparation | reactions |
Other organic reaction classification
In heterocyclic chemistry, organic reactions are classified by the type of heterocycle formed with respect to ring-size and type of heteroatom. See for instance the chemistry of indoleIndole
Indole is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. Indole is a popular component of fragrances and the precursor to many pharmaceuticals. Compounds that contain an...
s.
Organic reactions can also be classified by the type of bond to carbon with respect to the element involved. More reactions are found in organosilicon chemistry
Organosilicon
Organosilicon compounds are organic compounds containing carbon silicon bonds. Organosilicon chemistry is the corresponding science exploring their properties and reactivity.Like carbon, the organically bound silicon is tetravalent and tetrahedral...
, organosulfur chemistry, organophosphorus chemistry and organofluorine chemistry
Organofluorine chemistry
Organofluorine chemistry describes the chemistry of organofluorine compounds, organic compounds that contain the carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil- and water-repellents to pharmaceuticals, refrigerants and reagents in catalysis...
. With the introduction of carbon-metal bonds the field crosses over to organometallic chemistry
Organometallic chemistry
Organometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
.
See also
- List of organic reactions
- Other chemical reactions: inorganic reactions, metabolismMetabolismMetabolism is the set of chemical reactions that happen in the cells of living organisms to sustain life. These processes allow organisms to grow and reproduce, maintain their structures, and respond to their environments. Metabolism is usually divided into two categories...
, organometallic reactionOrganometallic chemistryOrganometallic chemistry is the study of chemical compounds containing bonds between carbon and a metal. Since many compounds without such bonds are chemically similar, an alternative may be compounds containing metal-element bonds of a largely covalent character...
s, polymerization reactions. - Important publications in organic chemistry

