Woodward-Hoffmann rules
Encyclopedia
The Woodward–Hoffmann rules devised by Robert Burns Woodward
and Roald Hoffmann
are a set of rules in organic chemistry
predicting the stereochemistry
of pericyclic reaction
s based on orbital symmetry. These include electrocyclic reaction
s, cycloaddition
s (including cheletropic reaction
s), sigmatropic reaction
s, and group transfer reaction
s. Hoffmann was awarded the 1981 Nobel Prize in Chemistry
for this work, shared with Kenichi Fukui
who developed a similar model; since Woodward had died two years before, he was not eligible to win what would have been his second Nobel Prize for Chemistry.
of electrocyclic
ring-opening and ring-closing reactions at the termini of open chain conjugated
polyene
s either by application of heat (thermal reactions) or application of light (photochemical
reactions). In the original publication in 1965, three rules are stated as:
Organic reactions that obey these rules are said to be symmetry allowed. Reactions that take the opposite course are symmetry forbidden and require a lot more energy to take place if they take place at all.
The rules predict the outcome of several ground-state reactions:
The stated rules are supported by theoretical calculations using the extended Hückel theory
. For example, the activation energy
required for thermal ring closing reaction of butadiene can be calculated as a function of the C-C-C bond angles keeping the other variables constant. Angles larger than 117° show a slight preference for a disrotatory
reaction but with smaller angles a conrotatory reaction mode is preferred.
A recent paper describes how mechanical stress can be used to reshape chemical reaction pathways to lead to products that apparently violate Woodward–Hoffman rules.
Here, (4q+2)s and (4r)a refer to suprafacial (4q+2)-electron and antarafacial (4r)-electron components, respectively. Alternatively, the general statement can be formulated as
involves an odd number of electron pairs (3 pairs of electrons, 2 pairs from the diene and 1 pair from the dienophile) and 0 antarafacial components (since there are 2 components and both are suprafacial). The conrotatory 4π thermal electrocyclization involves an even number of electron pairs (2 pairs) and 1 antarafacial component. Both reactions described above are thermally allowed processes. In practice, an even number of antarafacial components almost always means zero components, and an odd number almost always means one component, as transition states involving two or more antarafacial components are generally too strained to be feasible. Note that in this formulation, the electron count refers to the entire reacting system, rather than to individual components in Woodward and Hoffmann's original statement.
A pericyclic reaction in which these (equivalent) conditions are not satisfied is thermally forbidden. In general, reactions that are thermally forbidden are photochemically allowed, and vice versa.
, Corey makes his claim to priority of the idea: On May 4, 1964, I suggested to my colleague R. B. Woodward a simple explanation involving the symmetry of the perturbed (HOMO) molecular orbitals for the stereoselective cyclobutene to 1,3-butadiene and 1,3,5-hexatriene to cyclohexadiene conversions that provided the basis for the further development of these ideas into what became known as the Woodward–Hoffmann rules.
Corey, then 35, was working into the evening on Monday, May 4, as he and the other driven chemists often did. At about 8:30 p.m., he dropped by Woodward's office, and Woodward posed a question about how to predict the type of ring a chain of atoms would form. After some discussion, Corey proposed that the configuration of electrons governed the course of the reaction. Woodward insisted the solution wouldn't work, but Corey left drawings in the office, sure that he was on to something.
I felt that this was going to be a really interesting development and was looking forward to some sort of joint undertaking," he wrote. But the next day, Woodward flew into Corey's office as he and a colleague were leaving for lunch and presented Corey's idea as his own -- and then left. Corey was stunned.
In a 2004 rebuttal published in the Angewandte Chemie
, Roald Hoffmann
denied the claim: he quotes Woodward from a lecture given in 1966 saying: I REMEMBER very clearly—and it still surprises me somewhat—that the crucial flash of enlightenment came to me in algebraic, rather than in pictorial or geometric form. Out of the blue, it occurred to me that the coefficients of the terminal terms in the mathematical expression representing the highest occupied molecular orbital of butadiene were of opposite sign, while those of the corresponding expression for hexatriene possessed the same sign. From here it was but a short step to the geometric, and more obviously chemically relevant, view that in the internal cyclisation of a diene, the top face of one terminal atom should attack the bottom face of the other, while in the triene case, the formation of a new bond should involve the top (or pari passu, the bottom) faces of both terminal atoms.
In addition, Hoffmann points out that in 2 publications from 1963 and 1965, Corey described a total synthesis
of the compound dihydrocostunolide. Although in it an electrocyclic reaction is described, Corey has nothing to offer with respect to explaining its stereospecifity. Further, it is noteworthy that Corey said nothing of his so-called idea for nearly 2 decades after Woodward's death.
This photochemical reaction involving 4*1+2 electrons is now recognized as conrotatory.
Robert Burns Woodward
Robert Burns Woodward was an American organic chemist, considered by many to be the preeminent organic chemist of the twentieth century...
and Roald Hoffmann
Roald Hoffmann
Roald Hoffmann is an American theoretical chemist who won the 1981 Nobel Prize in Chemistry. He currently teaches at Cornell University in Ithaca, New York.-Escape from the Holocaust:...
are a set of rules in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
predicting the stereochemistry
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms within molecules. An important branch of stereochemistry is the study of chiral molecules....
of pericyclic reaction
Pericyclic reaction
In organic chemistry, a pericyclic reaction is a type of organic reaction wherein the transition state of the molecule has a cyclic geometry, and the reaction progresses in a concerted fashion. Pericyclic reactions are usually rearrangement reactions...
s based on orbital symmetry. These include electrocyclic reaction
Electrocyclic reaction
In organic chemistry, an electrocyclic reaction is a type of pericyclic rearrangement reaction where the net result is one pi bond being converted into one sigma bond or vice-versa...
s, cycloaddition
Cycloaddition
A cycloaddition is a pericyclic chemical reaction, in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity." The resulting reaction is a cyclization reaction.Cycloadditions are usually described by the...
s (including cheletropic reaction
Cheletropic reaction
thumb|300px|right|Pericyclic ReactionsCheletropic reactions are a type of pericyclic reaction. A pericyclic reaction is one that involves a transition state with a cyclic array of atoms and an associated cyclic array of interacting orbitals. A reorganization of σ and π bonds occurs in this cyclic...
s), sigmatropic reaction
Sigmatropic reaction
A sigmatropic reaction in organic chemistry is a pericyclic reaction wherein the net result is one σ-bond is changed to another σ-bond in an uncatalyzed intramolecular process. The name sigmatropic is the result of a compounding of the long-established sigma designation from single carbon-carbon...
s, and group transfer reaction
Group transfer reaction
Group transfer reactions are pericyclic reactions where one pi bond is converted to one sigma bond, at the same time that a sigma bond migrates.The best known group transfer reaction is the ene reaction....
s. Hoffmann was awarded the 1981 Nobel Prize in Chemistry
Nobel Prize in Chemistry
The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outstanding contributions in chemistry, physics, literature,...
for this work, shared with Kenichi Fukui
Kenichi Fukui
Kenichi Fukui was a Japanese chemist.Kenichi Fukui was co-recipient of the Nobel Prize in Chemistry in 1981 with Roald Hoffmann, for their independent investigations into the mechanisms of chemical reactions...
who developed a similar model; since Woodward had died two years before, he was not eligible to win what would have been his second Nobel Prize for Chemistry.
Electrocyclic reaction
The rules apply to the observed stereospecificityStereospecificity
In chemistry, stereospecificity is the property of a reaction mechanism that leads to different stereoisomeric reaction products from different stereoisomeric reactants, or which operates on only one of the stereoisomers."Overlap Control of Carbanionoid Reactions. I. Stereoselectivity in Alkaline...
of electrocyclic
Electrocyclic reaction
In organic chemistry, an electrocyclic reaction is a type of pericyclic rearrangement reaction where the net result is one pi bond being converted into one sigma bond or vice-versa...
ring-opening and ring-closing reactions at the termini of open chain conjugated
Conjugated system
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. Lone pairs, radicals or carbenium ions may be part of the...
polyene
Polyene
Polyenes are poly-unsaturated organic compounds that contain one or more sequences of alternating double and single carbon-carbon bonds. These double carbon-carbon bonds interact in a process known as conjugation, which results in an overall lower energy state of the molecule.Organic compounds with...
s either by application of heat (thermal reactions) or application of light (photochemical
Photochemistry
Photochemistry, a sub-discipline of chemistry, is the study of chemical reactions that proceed with the absorption of light by atoms or molecules.. Everyday examples include photosynthesis, the degradation of plastics and the formation of vitamin D with sunlight.-Principles:Light is a type of...
reactions). In the original publication in 1965, three rules are stated as:
- In an open-chain system containing 4n-electronsAntiaromaticityAntiaromatic molecules are cyclic systems containing alternating single and double bonds, where the pi electron energy of antiaromatic compounds is higher than that of its open-chain counterpart. Therefore antiaromatic compounds are unstable and highly reactive; often antiaromatic compounds...
, the orbital symmetry of the highest occupied ground-state orbitalHomoHomo may refer to:*the Greek prefix ὅμο-, meaning "the same"*the Latin for man, human being*Homo, the taxonomical genus including modern humans...
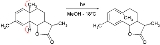
is such that a bonding interaction between the termini must involve overlap between orbital envelopes on opposite faces of the system and this can only be achieved in a conrotatory process. An example of such reaction type is the Nazarov cyclization reactionNazarov cyclization reactionThe Nazarov cyclization reaction is a chemical reaction used in organic chemistry for the synthesis of cyclopentenones. The reaction is typically divided into classical and modern variants, depending on the reagents and substrates employed...
of divinylketones.
- In open systems containing 4n + 2 electronsAromaticityIn organic chemistry, Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. The earliest use of the term was in an article by August...
, terminal bonding interaction within ground-state molecules requires overlap of orbital envelopes on the same face of the system, attainable only by disrotatoryDisrotatoryIn a conrotatory mode of an electrocyclic reaction the substituents located at the termini of a conjugated double bond system move in the same direction during ring opening or ring closure...
displacements.
- In a photochemical reaction an electron in the HOMOHomoHomo may refer to:*the Greek prefix ὅμο-, meaning "the same"*the Latin for man, human being*Homo, the taxonomical genus including modern humans...
of the reactant is promoted to an excited stateExcited stateExcitation is an elevation in energy level above an arbitrary baseline energy state. In physics there is a specific technical definition for energy level which is often associated with an atom being excited to an excited state....
leading to a reversal of terminal symmetry relationships and reversal of stereospecificity.
Organic reactions that obey these rules are said to be symmetry allowed. Reactions that take the opposite course are symmetry forbidden and require a lot more energy to take place if they take place at all.
The rules predict the outcome of several ground-state reactions:
- CyclopropylCyclopropaneCyclopropane is a cycloalkane molecule with the molecular formula C3H6, consisting of three carbon atoms linked to each other to form a ring, with each carbon atom bearing two hydrogen atoms...
cation → allylAllylAn allyl group is a substituent with the structural formula H2C=CH-CH2R, where R is the connection to the rest of the molecule. It is made up of a methylene , attached to a vinyl group . The name is derived from the Latin word for garlic, Allium sativum. Theodor Wertheim isolated an allyl...
cation: disrotatory - Cyclopropyl radicalRadical (chemistry)Radicals are atoms, molecules, or ions with unpaired electrons on an open shell configuration. Free radicals may have positive, negative, or zero charge...
→ allyl radical: conrotatory - Cyclopropyl anion → allyl anion: conrotatory
- CyclopentenylCyclopentadieneCyclopentadiene is an organic compound with the formula C5H6. This colorless liquid has a strong and unpleasant odor. At room temperature, this cyclic diene dimerizes over the course of hours to give dicyclopentadiene via a Diels–Alder reaction...
cation → pentadienyl cation: conrotatory
The stated rules are supported by theoretical calculations using the extended Hückel theory
Extended Huckel method
The extended Hückel method is a semiempirical quantum chemistry method, developed by Roald Hoffmann since 1963. It is based on the Hückel method but, while the original Hückel method only considers pi orbitals, the extended method also includes the sigma orbitals.The extended Hückel method can be...
. For example, the activation energy
Activation energy
In chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
required for thermal ring closing reaction of butadiene can be calculated as a function of the C-C-C bond angles keeping the other variables constant. Angles larger than 117° show a slight preference for a disrotatory
Disrotatory
In a conrotatory mode of an electrocyclic reaction the substituents located at the termini of a conjugated double bond system move in the same direction during ring opening or ring closure...
reaction but with smaller angles a conrotatory reaction mode is preferred.
A recent paper describes how mechanical stress can be used to reshape chemical reaction pathways to lead to products that apparently violate Woodward–Hoffman rules.
General Formulation
Though the Woodward–Hoffmann rules were first stated in terms of electrocyclic processes, they were soon generalized to all pericyclic reactions. In the generalized Woodward-Hoffmann rules the more inclusive bond topology descriptors antarafacial and suprafacial subsume the terms conrotatory and disrotatory, respectively. Antarafacial refers to bond making or breaking through the opposite face of a π system, p orbital, or σ bond, while suprafacial refers to the process occurring through the same face. The general statement, as given in Woodward and Hoffmann's 1969 review is as follows:A ground-state pericyclic change is symmetry-allowed when the total number of (4q+2)s and (4r)a components is odd.
Here, (4q+2)s and (4r)a refer to suprafacial (4q+2)-electron and antarafacial (4r)-electron components, respectively. Alternatively, the general statement can be formulated as
A pericyclic reaction involving 4n+2 or 4n electrons is thermally allowed if the number of antarafacial components is even or odd, respectively.In other words, a pericyclic reaction is allowed if the sum of the number of electron pairs involved and number of antarafacial components is odd. For example, the Diels-Alder reaction
Diels-Alder reaction
The Diels–Alder reaction is an organic chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. The reaction can proceed even if some of the atoms in the newly formed ring are not carbon...
involves an odd number of electron pairs (3 pairs of electrons, 2 pairs from the diene and 1 pair from the dienophile) and 0 antarafacial components (since there are 2 components and both are suprafacial). The conrotatory 4π thermal electrocyclization involves an even number of electron pairs (2 pairs) and 1 antarafacial component. Both reactions described above are thermally allowed processes. In practice, an even number of antarafacial components almost always means zero components, and an odd number almost always means one component, as transition states involving two or more antarafacial components are generally too strained to be feasible. Note that in this formulation, the electron count refers to the entire reacting system, rather than to individual components in Woodward and Hoffmann's original statement.
A pericyclic reaction in which these (equivalent) conditions are not satisfied is thermally forbidden. In general, reactions that are thermally forbidden are photochemically allowed, and vice versa.
Controversy
It has been stated that the chemist E. J. Corey, also a Nobel Prize winner, feels he is responsible for the ideas that laid the foundation for this research, and that Woodward unfairly neglected to credit him in the discovery. In a 2004 memoir published in the Journal of Organic ChemistryJournal of Organic Chemistry
The Journal of Organic Chemistry is a peer-reviewed scientific journal for original contributions of fundamental research in organic and bioorganic chemistry. It is published by the American Chemical Society. Its 2010 impact factor is 4.002....
, Corey makes his claim to priority of the idea: On May 4, 1964, I suggested to my colleague R. B. Woodward a simple explanation involving the symmetry of the perturbed (HOMO) molecular orbitals for the stereoselective cyclobutene to 1,3-butadiene and 1,3,5-hexatriene to cyclohexadiene conversions that provided the basis for the further development of these ideas into what became known as the Woodward–Hoffmann rules.
Corey, then 35, was working into the evening on Monday, May 4, as he and the other driven chemists often did. At about 8:30 p.m., he dropped by Woodward's office, and Woodward posed a question about how to predict the type of ring a chain of atoms would form. After some discussion, Corey proposed that the configuration of electrons governed the course of the reaction. Woodward insisted the solution wouldn't work, but Corey left drawings in the office, sure that he was on to something.
I felt that this was going to be a really interesting development and was looking forward to some sort of joint undertaking," he wrote. But the next day, Woodward flew into Corey's office as he and a colleague were leaving for lunch and presented Corey's idea as his own -- and then left. Corey was stunned.
In a 2004 rebuttal published in the Angewandte Chemie
Angewandte Chemie
Angewandte Chemie is a weekly peer-reviewed scientific journal that covers all aspects of chemistry. Its impact factor was 12.730 in 2010, the highest value for a chemistry-specific journal that publishes original research...
, Roald Hoffmann
Roald Hoffmann
Roald Hoffmann is an American theoretical chemist who won the 1981 Nobel Prize in Chemistry. He currently teaches at Cornell University in Ithaca, New York.-Escape from the Holocaust:...
denied the claim: he quotes Woodward from a lecture given in 1966 saying: I REMEMBER very clearly—and it still surprises me somewhat—that the crucial flash of enlightenment came to me in algebraic, rather than in pictorial or geometric form. Out of the blue, it occurred to me that the coefficients of the terminal terms in the mathematical expression representing the highest occupied molecular orbital of butadiene were of opposite sign, while those of the corresponding expression for hexatriene possessed the same sign. From here it was but a short step to the geometric, and more obviously chemically relevant, view that in the internal cyclisation of a diene, the top face of one terminal atom should attack the bottom face of the other, while in the triene case, the formation of a new bond should involve the top (or pari passu, the bottom) faces of both terminal atoms.
In addition, Hoffmann points out that in 2 publications from 1963 and 1965, Corey described a total synthesis
Total synthesis
In organic chemistry, a total synthesis is, in principle, the complete chemical synthesis of complex organic molecules from simpler pieces, usually without the aid of biological processes. In practice, these simpler pieces are commercially available in bulk and semi-bulk quantities, and are often...
of the compound dihydrocostunolide. Although in it an electrocyclic reaction is described, Corey has nothing to offer with respect to explaining its stereospecifity. Further, it is noteworthy that Corey said nothing of his so-called idea for nearly 2 decades after Woodward's death.
This photochemical reaction involving 4*1+2 electrons is now recognized as conrotatory.
External links
- Symmetry rules!, Sophie Wilkinson Chemical & Engineering NewsChemical & Engineering NewsChemical & Engineering News is a weekly magazine published by the American Chemical Society, providing professional and technical information in the fields of chemistry and chemical engineering...
January 27, 2003 Volume 81, Number 04 CENEAR 81 04 pp. 59 ISSN 0009-2347 Article - "SHMO calculator", Simple Huckel molecular orbital theory calculator Link


