
Electrocyclic reaction
Encyclopedia
In organic chemistry
, an electrocyclic reaction is a type of pericyclic rearrangement reaction
where the net result is one pi bond
being converted into one sigma bond
or vice-versa. These reactions are usually unnamed, being categorized by the following criteria:
The torquoselectivity
in an electrocyclic reaction refers to the direction of rotation. For example, a reaction that is conrotatory can still rotate in two directions, producing enantiomeric products. A reaction that is torquoselective restricts one of these directions of rotation (partially or completely) to produce a product in enantiomeric excess
.
The Nazarov cyclization reaction
is a named electrocyclic reaction converting divinylketones to cyclopentenones.
A classic example is the thermal ring-opening reaction of 3,4-dimethylcyclobutene. The cis isomer exclusively yields cis,trans-2,4-hexadiene whereas the trans isomer gives the trans,trans diene :

This reaction course can be explained in a simple analysis through the frontier-orbital method: the sigma bond in the reactant will open in such a way that the resulting p-orbitals will have the same symmetry as the HOMO
of the product (a butadiene). The only way to accomplish this is through a conrotatory ring-opening which results in opposite signs for the terminal lobes.
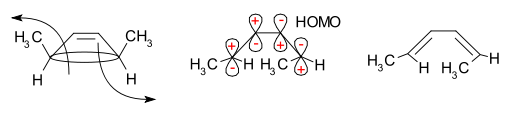
The stereospecificity
is then determined by whether the reaction proceeds through a conrotatory or disrotatory process.

Correlation diagrams, which connect the molecular orbitals of the reactant to those of the product having the same symmetry, can then be constructed for the two processes.
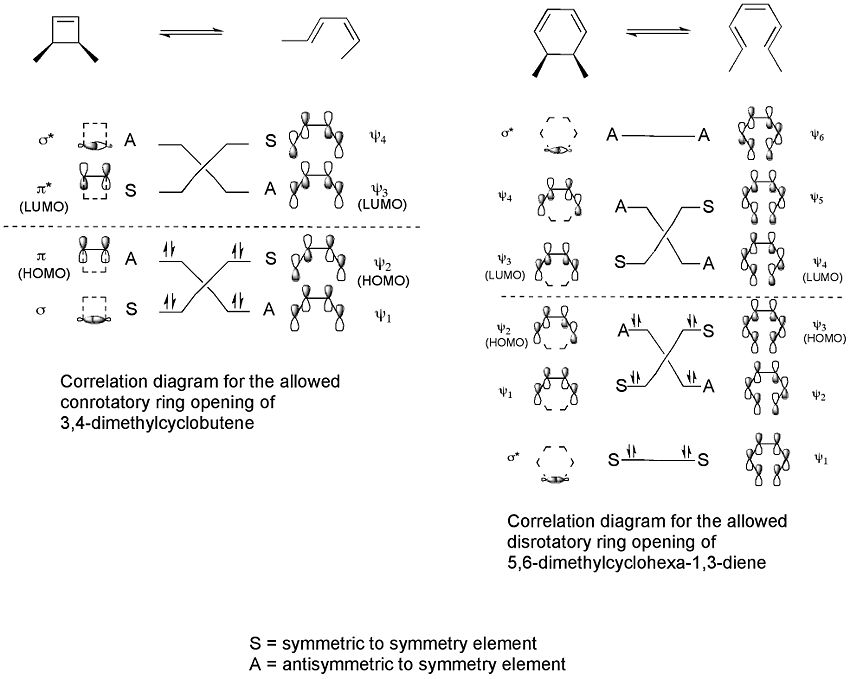
These correlation diagrams indicate that only a conrotatory ring opening of 3,4-dimethylcyclobutene is symmetry allowed whereas only a disrotatory ring opening of 5,6-dimethylcyclohexa-1,3-diene is symmetry allowed. This is because only in these cases would maximum orbital overlap occur in the transition state. Also, the formed product would be in a ground state rather than an excited state.

For the 5,6-dimethylcyclohexa-1,3-diene, only a disrotatory mode would result in p-orbitals having the same symmetry as the HOMO of hexatriene. For the 3,4-dimethylcyclobutene, on the other hand, only a conrotatory mode would result in p-orbitals having the same symmetry as the HOMO of butadiene.
Only a disrotatory mode, in which symmetry about a reflection plane is maintained throughout the reaction, would result in maximum orbital overlap in the transition state. Also, once again, this would result in the formation of a product that is in an excited state of comparable stability to the excited state of the reactant compound.
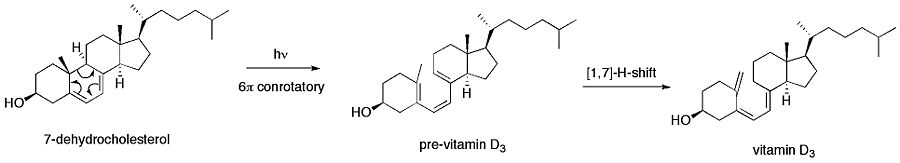
The first step involves a photochemically induced conrotatory ring opening of 7-dehydrocholesterol to form pre vitamin D3. A [1,7]-hydride shift then forms vitamin D3.
Another example is in the proposed biosynthesis of aranotin, a naturally occurring oxepine, and its related compounds.
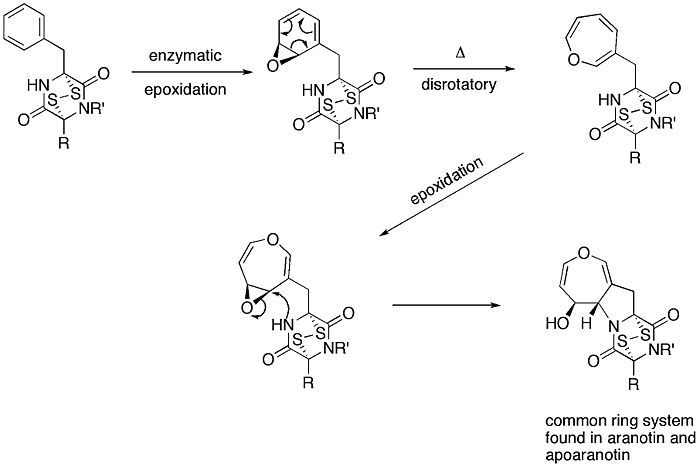
Enzymatic epoxidation of phenylalanine-derived diketopiperazine forms the arene oxide, which undergoes a 6π disrotatory ring opening electrocyclization reaction to produce the uncyclized oxepine. After a second epoxidation of the ring, the nearby nucleophilic nitrogen attacks the electrophilic carbon, forming a five membered ring. The resulting ring system is a common ring system found in aranotin and its related compounds.
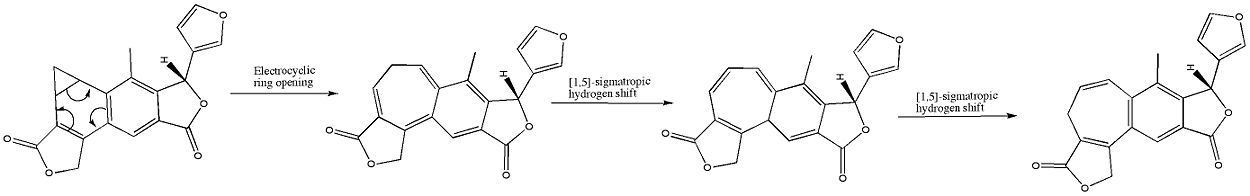
The benzonorcaradiene diterpenoid (A) was rearranged into the benzocycloheptatriene diterpenoid isosalvipuberlin (B) by boiling a methylene chloride solution. This transformation can be envisaged as a disrotatory electrocyclic reaction, followed by two suprafacial 1,5-simatropic hydrogen shifts, as shown bellow.
to the Diels-Alder adduct. The chemical yield for the ring opening of the benzocyclobutane depicted in scheme 2 is found to depend on the nature of the substituent
R. With a reaction solvent
such as toluene
and a reaction temperature of 110°C, the yield increases going from methyl to isobutylmethyl to trimethylsilylmethyl. The increased reaction rate
for the trimethylsilyl
compound can be explained by silicon hyperconjugation as the βC-Si bond weakens the cyclobutane C-C bond by donating electrons.
 An biomimetic electrocyclic cascade reaction
An biomimetic electrocyclic cascade reaction
was discovered in relation to the isolation and synthesis of certain endiandric acids :
 Asymmetric electrocyclic reactions are an emerging field in contemporary organic synthesis. The most commonly-studied reactions in this field are the 4π Staudinger β-lactam synthesis and the 4π Nazarov reaction; asymmetric catalysis of both reactions have been controlled by use of a chiral auxiliary
Asymmetric electrocyclic reactions are an emerging field in contemporary organic synthesis. The most commonly-studied reactions in this field are the 4π Staudinger β-lactam synthesis and the 4π Nazarov reaction; asymmetric catalysis of both reactions have been controlled by use of a chiral auxiliary
, and the Nazarov reaction has been performed catalytically
using chiral Lewis acids, Brønsted acids and chiral amines.
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
, an electrocyclic reaction is a type of pericyclic rearrangement reaction
Rearrangement reaction
A rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule...
where the net result is one pi bond
Pi bond
In chemistry, pi bonds are covalent chemical bonds where two lobes of one involved atomic orbital overlap two lobes of the other involved atomic orbital...
being converted into one sigma bond
Sigma bond
In chemistry, sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most clearly defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a σ-bond is...
or vice-versa. These reactions are usually unnamed, being categorized by the following criteria:
- electrocyclic reactions are photoinducedPhotochemistryPhotochemistry, a sub-discipline of chemistry, is the study of chemical reactions that proceed with the absorption of light by atoms or molecules.. Everyday examples include photosynthesis, the degradation of plastics and the formation of vitamin D with sunlight.-Principles:Light is a type of...
or thermalThermalA thermal column is a column of rising air in the lower altitudes of the Earth's atmosphere. Thermals are created by the uneven heating of the Earth's surface from solar radiation, and are an example of convection. The sun warms the ground, which in turn warms the air directly above it... - the number of pi electrons in the species with more pi bonds determines reaction mode
- electrocyclic reaction can be a ring closure (electrocyclization) or a ring opening reaction
- the stereospecifity is determined by conrotatory or disrotatoryDisrotatoryIn a conrotatory mode of an electrocyclic reaction the substituents located at the termini of a conjugated double bond system move in the same direction during ring opening or ring closure...
mode of transition stateTransition stateThe transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest energy along this reaction coordinate. At this point, assuming a perfectly irreversible reaction, colliding reactant molecules will always...
formation as predicted by the Woodward-Hoffmann rulesWoodward-Hoffmann rulesThe Woodward–Hoffmann rules devised by Robert Burns Woodward and Roald Hoffmann are a set of rules in organic chemistry predicting the stereochemistry of pericyclic reactions based on orbital symmetry. These include electrocyclic reactions, cycloadditions , sigmatropic reactions, and group transfer...
.
The torquoselectivity
Torquoselectivity
Torquoselectivity is a special kind of stereoselectivity observed in electrocyclic reactions in organic chemistry, defined as "the preference for inward or outward rotation of substituents in conrotatory...
in an electrocyclic reaction refers to the direction of rotation. For example, a reaction that is conrotatory can still rotate in two directions, producing enantiomeric products. A reaction that is torquoselective restricts one of these directions of rotation (partially or completely) to produce a product in enantiomeric excess
Enantiomeric excess
The enantiomeric excess of a substance is a measure of how pure it is. In this case, the impurity is the undesired enantiomer .-Definition:...
.
The Nazarov cyclization reaction
Nazarov cyclization reaction
The Nazarov cyclization reaction is a chemical reaction used in organic chemistry for the synthesis of cyclopentenones. The reaction is typically divided into classical and modern variants, depending on the reagents and substrates employed...
is a named electrocyclic reaction converting divinylketones to cyclopentenones.
A classic example is the thermal ring-opening reaction of 3,4-dimethylcyclobutene. The cis isomer exclusively yields cis,trans-2,4-hexadiene whereas the trans isomer gives the trans,trans diene :

This reaction course can be explained in a simple analysis through the frontier-orbital method: the sigma bond in the reactant will open in such a way that the resulting p-orbitals will have the same symmetry as the HOMO
Homo
Homo may refer to:*the Greek prefix ὅμο-, meaning "the same"*the Latin for man, human being*Homo, the taxonomical genus including modern humans...
of the product (a butadiene). The only way to accomplish this is through a conrotatory ring-opening which results in opposite signs for the terminal lobes.
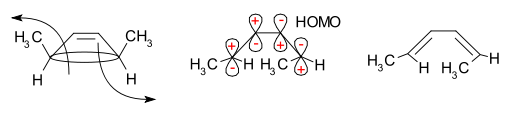
| system | Thermally Induced (ground state) | Photochemically Induced (excited state) |
|---|---|---|
| "4n" e- | Conrotatory | Disrotatory |
| "4n + 2" e- | Disrotatory | Conrotatory |
The stereospecificity
Stereospecificity
In chemistry, stereospecificity is the property of a reaction mechanism that leads to different stereoisomeric reaction products from different stereoisomeric reactants, or which operates on only one of the stereoisomers."Overlap Control of Carbanionoid Reactions. I. Stereoselectivity in Alkaline...
is then determined by whether the reaction proceeds through a conrotatory or disrotatory process.
Woodward-Hoffman Rules

Correlation diagrams, which connect the molecular orbitals of the reactant to those of the product having the same symmetry, can then be constructed for the two processes.

These correlation diagrams indicate that only a conrotatory ring opening of 3,4-dimethylcyclobutene is symmetry allowed whereas only a disrotatory ring opening of 5,6-dimethylcyclohexa-1,3-diene is symmetry allowed. This is because only in these cases would maximum orbital overlap occur in the transition state. Also, the formed product would be in a ground state rather than an excited state.
Frontier molecular orbital Theory
According to the Frontier Molecular Orbital Theory, the sigma bond in the ring will open in such a way that the resulting p-orbitals will have the same symmetry as the HOMO of the product.
For the 5,6-dimethylcyclohexa-1,3-diene, only a disrotatory mode would result in p-orbitals having the same symmetry as the HOMO of hexatriene. For the 3,4-dimethylcyclobutene, on the other hand, only a conrotatory mode would result in p-orbitals having the same symmetry as the HOMO of butadiene.
Excited state electrocyclizations
If the ring opening of 3,4-dimethylcyclobutene were carried out under photochemical conditions the resulting electrocyclization would be occur via a disrotatory mode instead of a conrotatory mode as can be seen by the correlation diagram for the allowed excited state ring opening reaction.Only a disrotatory mode, in which symmetry about a reflection plane is maintained throughout the reaction, would result in maximum orbital overlap in the transition state. Also, once again, this would result in the formation of a product that is in an excited state of comparable stability to the excited state of the reactant compound.
Electrocyclic Reactions in Biological Systems
Electrocyclic reactions occur frequently in nature. One of the most common such electrocyclizations is the biosynthesis of vitamin D3.
The first step involves a photochemically induced conrotatory ring opening of 7-dehydrocholesterol to form pre vitamin D3. A [1,7]-hydride shift then forms vitamin D3.
Another example is in the proposed biosynthesis of aranotin, a naturally occurring oxepine, and its related compounds.

Enzymatic epoxidation of phenylalanine-derived diketopiperazine forms the arene oxide, which undergoes a 6π disrotatory ring opening electrocyclization reaction to produce the uncyclized oxepine. After a second epoxidation of the ring, the nearby nucleophilic nitrogen attacks the electrophilic carbon, forming a five membered ring. The resulting ring system is a common ring system found in aranotin and its related compounds.
The benzonorcaradiene diterpenoid (A) was rearranged into the benzocycloheptatriene diterpenoid isosalvipuberlin (B) by boiling a methylene chloride solution. This transformation can be envisaged as a disrotatory electrocyclic reaction, followed by two suprafacial 1,5-simatropic hydrogen shifts, as shown bellow.

Scope
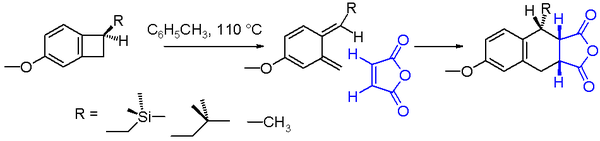
An often studied electrocyclic reaction is the conrotatory thermal ring-opening of benzocyclobutane. The reaction product is a very unstable ortho-quinodimethane but this molecule can be trapped in an endo addition with a strong dienophile such as maleic anhydrideMaleic anhydride
Maleic anhydride is an organic compound with the formula C2H22O. It is the acid anhydride of maleic acid and in its pure state it is a colourless or white solid with an acrid odour....
to the Diels-Alder adduct. The chemical yield for the ring opening of the benzocyclobutane depicted in scheme 2 is found to depend on the nature of the substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
R. With a reaction solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
such as toluene
Toluene
Toluene, formerly known as toluol, is a clear, water-insoluble liquid with the typical smell of paint thinners. It is a mono-substituted benzene derivative, i.e., one in which a single hydrogen atom from the benzene molecule has been replaced by a univalent group, in this case CH3.It is an aromatic...
and a reaction temperature of 110°C, the yield increases going from methyl to isobutylmethyl to trimethylsilylmethyl. The increased reaction rate
Reaction rate
The reaction rate or speed of reaction for a reactant or product in a particular reaction is intuitively defined as how fast or slow a reaction takes place...
for the trimethylsilyl
Trimethylsilyl
A trimethylsilyl group is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom [−Si3], which is in turn bonded to the rest of a molecule...
compound can be explained by silicon hyperconjugation as the βC-Si bond weakens the cyclobutane C-C bond by donating electrons.

Cascade reaction
A cascade reaction or tandem reaction or domino reaction is a consecutive series of intramolecular organic reactions which often proceed via highly reactive intermediates. It allows the organic synthesis of complex multinuclear molecules from a single acyclic precursor. The substrate contains many...
was discovered in relation to the isolation and synthesis of certain endiandric acids :

Chiral auxiliary
A chiral auxiliary is a chemical compound or unit that is temporarily incorporated into an organic synthesis so that it can be carried out asymmetrically with the selective formation of one of two enantiomers...
, and the Nazarov reaction has been performed catalytically
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
using chiral Lewis acids, Brønsted acids and chiral amines.

