
Rearrangement reaction
Encyclopedia
A rearrangement reaction is a broad class of organic reaction
s where the carbon skeleton of a molecule
is rearranged to give a structural isomer of the original molecule. Often a substituent
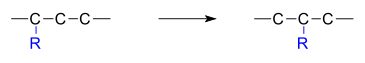
moves from one atom to another atom in the same molecule. In the example below the substituent R moves from carbon atom 1 to carbon atom 2:
 Intermolecular rearrangements also take place.
Intermolecular rearrangements also take place.
A rearrangement is not well represented by simple and discrete electron transfers (represented by curly arrows in organic chemistry texts). The actual mechanism of alkyl groups moving, as in Wagner-Meerwein rearrangement
, probably involves transfer of the moving alkyl group fluidly along a bond, not ionic bond-breaking and forming. In pericyclic reactions, explanation by orbital interactions give a better picture than simple discrete electron transfers. It is, nevertheless, possible to draw the curved arrows for a sequence of discrete electron transfers that give the same result as a rearrangement reaction, although these are not necessarily realistic. In allylic rearrangement
, the reaction is indeed ionic.
Three key rearrangement reactions are 1,2-rearrangement
s, pericyclic reactions and olefin metathesis
.
:
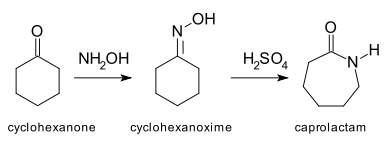
 and the Beckmann rearrangement
and the Beckmann rearrangement
:
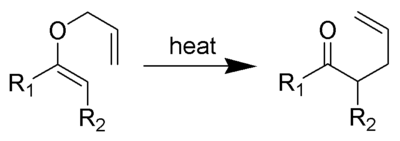
 and the Claisen rearrangement
and the Claisen rearrangement
:
, or more accurately, transition metal carbene complex
intermediates.
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
s where the carbon skeleton of a molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
is rearranged to give a structural isomer of the original molecule. Often a substituent
Substituent
In organic chemistry and biochemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon...
moves from one atom to another atom in the same molecule. In the example below the substituent R moves from carbon atom 1 to carbon atom 2:

A rearrangement is not well represented by simple and discrete electron transfers (represented by curly arrows in organic chemistry texts). The actual mechanism of alkyl groups moving, as in Wagner-Meerwein rearrangement
Wagner-Meerwein rearrangement
A Wagner–Meerwein rearrangement is a class of carbocation 1,2-rearrangement reactions in which a hydrogen, alkyl or aryl group migrates from one carbon to a neighboring carbon.Several reviews have been published....
, probably involves transfer of the moving alkyl group fluidly along a bond, not ionic bond-breaking and forming. In pericyclic reactions, explanation by orbital interactions give a better picture than simple discrete electron transfers. It is, nevertheless, possible to draw the curved arrows for a sequence of discrete electron transfers that give the same result as a rearrangement reaction, although these are not necessarily realistic. In allylic rearrangement
Allylic rearrangement
An allylic rearrangement or allylic shift is an organic reaction in which the double bond in an allyl chemical compound shifts to the next carbon atom. It is encountered in nucleophilic substitution....
, the reaction is indeed ionic.
Three key rearrangement reactions are 1,2-rearrangement
1,2-rearrangement
A 1,2-rearrangement or 1,2-migration or 1,2-shift or Whitmore 1,2-shift is an organic reaction where a substituent moves from one atom to another atom in a chemical compound. In a 1,2 shift the movement involves two adjacent atoms but moves over larger distances are possible...
s, pericyclic reactions and olefin metathesis
Olefin metathesis
Olefin metathesis or transalkylidenation is an organic reaction that entails redistribution of alkylene fragments by the scission of carbon - carbon double bonds in olefins . Its advantages include the creation of fewer sideproducts and hazardous wastes. Yves Chauvin, Robert H. Grubbs, and Richard R...
.
1,2-rearrangements
A 1,2-rearrangement is an organic reaction where a substituent moves from one atom to another atom in a chemical compound. In a 1,2 shift the movement involves two adjacent atoms but moves over larger distances are possible. Examples are the Wagner-Meerwein rearrangementWagner-Meerwein rearrangement
A Wagner–Meerwein rearrangement is a class of carbocation 1,2-rearrangement reactions in which a hydrogen, alkyl or aryl group migrates from one carbon to a neighboring carbon.Several reviews have been published....
:

Beckmann rearrangement
The Beckmann rearrangement, named after the German chemist Ernst Otto Beckmann , is an acid-catalyzed rearrangement of an oxime to an amide...
:

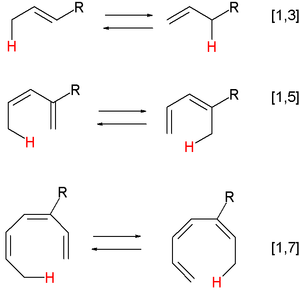
Pericyclic reactions
A pericyclic reaction is a type of reaction with multiple carbon-carbon bond making and breaking wherein the transition state of the molecule has a cyclic geometry, and the reaction progresses in a concerted fashion. Examples are hydride shifts
Claisen rearrangement
The Claisen rearrangement is a powerful carbon-carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen...
:

Olefin metathesis
Olefin metathesis is a formal exchange of the alkylidene fragments in two alkenes. It is a catalytic reaction with carbeneCarbene
In chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is RR'C:, but the carbon can instead be double-bonded to one group. The term "carbene" may also merely refer to the compound H2C:, also called...
, or more accurately, transition metal carbene complex
Transition metal carbene complex
A transition metal carbene complex is a organometallic compound featuring a divalent organic ligand. The divalent organic ligand coordinated to the metal center is called a carbene. Carbene complexes for almost all transition metals have been reported. Many methods for synthesizing them and...
intermediates.
See also
- Beckmann rearrangementBeckmann rearrangementThe Beckmann rearrangement, named after the German chemist Ernst Otto Beckmann , is an acid-catalyzed rearrangement of an oxime to an amide...
- Curtius rearrangementCurtius rearrangementThe Curtius rearrangement , as first defined by Theodor Curtius, is a chemical reaction that involves the rearrangement of an acyl azide to an isocyanate. Several reviews have been published....
- Hofmann rearrangementHofmann rearrangementThe Hofmann rearrangement is the organic reaction of a primary amide to a primary amine with one fewer carbon atom.The reaction is named after its discoverer: August Wilhelm von Hofmann...
- Lossen rearrangementLossen rearrangementThe Lossen rearrangement is the conversion of a hydroxamic acid to an isocyanate via the formation of an O-acyl, sulfonyl, or phosphoryl intermediate hydroxamic acid O-derivative and then conversion to its conjugate base. Here, 4-Toluenesulfonyl chloride is used to form a sulfonyl O-derivative...
- Schmidt reactionSchmidt reactionThe Schmidt reaction is an organic reaction involving alkyl migration over the carbon-nitrogen chemical bond in an azide with expulsion of nitrogen...
- Tiemann rearrangement
- Wolff rearrangementWolff rearrangementThe Wolff rearrangement is a rearrangement reaction converting a α-diazo-ketone into a ketene. This reaction was first reported by Ludwig Wolff in 1912....
- Photochemical rearrangementsMechanistic organic photochemistryMechanistic organic photochemistry is that aspect of organic photochemistry which seeks to explain the mechanisms of organic photochemical reactions. The absorption of ultraviolet light by organic molecules very often leads to reactions. In the earliest days sunlight was employed while in more...

