Olefin metathesis
Encyclopedia
Olefin metathesis or transalkylidenation is an organic reaction
that entails redistribution of alkylene fragments by the scission of carbon - carbon double bonds
in olefins (alkenes). Its advantages include the creation of fewer sideproducts and hazardous waste
s. Yves Chauvin
, Robert H. Grubbs
, and Richard R. Schrock
shared the 2005 Nobel Prize in Chemistry
for "the development of the metathesis method in organic synthesis
".
The reaction is catalyzed
by metals such as nickel
, tungsten
, rhenium
, ruthenium
and molybdenum
. The reaction consists of an alkene
double bond
cleavage
, followed by a statistical redistribution of alkylidene fragments. The general scope is outlined by the following scheme:
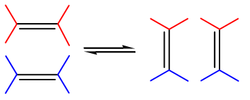
reformation for the synthesis of higher olefins from the products (α-olefins) from the Shell higher olefin process
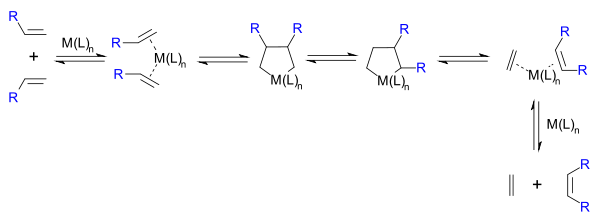
(SHOP) under high pressure and high temperatures. Traditional catalysts are derived from a reaction of the metal halides with alkylation agents for example WCl6-EtOH-EtAlCl2. A metathesis reaction is a chain reaction that begins when a metallocarbene and an olefin react to form a metallacyclobutane. This intermediate then reacts further, decomposing into a new olefin (the product) and a new metallocarbene, which can then be recycled through the reaction pathway.
 The Grubbs' catalyst
The Grubbs' catalyst
is a ruthenium carbenoid, whereas molybdenum(VI) and tungsten(VI)-based catalysts are known as Schrock alkylidene
s. Related complexes can also catalyze alkyne metathesis
and related polymerizations.
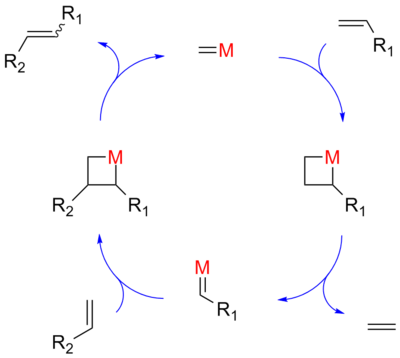
and thus has a high activation energy
. The Chauvin mechanism involves the [2+2] cycloaddition of an alkene double bond to a transition metal alkylidene to form a metallocyclobutane intermediate. The metallocyclobutane produced can then cyclorevert to give either the original species or a new alkene and alkylidene. Interaction with the d-orbitals
on the metal catalyst lowers the activation energy enough that the reaction can proceed rapidly at modest temperatures.
chemistry:
Like most chemical reactions, the metathesis pathway is usually driven by a thermodynamic imperative; that is, the final products are determined by the energetics of the possible products, with a distribution of products proportional to the exponential of their respective energy values.
Alkene metathesis is often generally driven by the evolution of ethylene
, and alkyne metathesis is driven by the evolution of acetylene
, which are both gases; hence, metathesis reactions, if they are not ring-opening or ring-closing, usually use monosubstituted and 2,2-disubstituted alkenes and terminal alkynes. These are both dominated by the entropy gained by the net release of gas. Enyne
metathesis cannot evolve a simple gas, and for that reason is usually disfavored unless there are accompanying ring-opening or ring-closing advantages. Ring opening metathesis usually involves a strained alkene (often a norbornene
) and the release of ring strain drives the reaction. Ring-closing metathesis
, conversely, usually involves the formation of a five- or six-membered ring which is highly energetically favorable; although these reactions tend to also evolve ethylene
. RCM has been used to close larger macrocycles, in which case the reaction may be kinetically controlled by running the reaction at extreme dilutions. The Thorpe-Ingold effect
may be exploited to improve both reaction rates and selectivity.
Alkene metathesis is synthetically equivalent to (and has replaced) a procedure of ozonolysis
of an alkene to two ketone fragments followed by the reaction of one of them with a Wittig reagent.
 The metathesis reaction of 1-hexene
The metathesis reaction of 1-hexene
with the WCl4(OAr)2 catalyst yields 5-decene plus many byproducts from secondary metathesis reactions.
in the 1950s who as part of ongoing work in what would later become known as Ziegler-Natta catalysis studied ethylene
polymerization
which on addition of certain metals resulted in 1-butene
instead of a saturated long-chain hydrocarbon (see nickel effect) .
In 1960 a Du Pont research group polymerized norbornene
to polynorbornene using lithium aluminum tetraheptyl and titanium tetrachloride
(a patent by this company on this topic dates back to 1955 ),
a reaction then classified as a so-called coordination polymerization
. According to the then proposed reaction mechanism
a RTiX titanium intermediate first coordinates to the double bond in a pi complex. The second step then is a concerted SNi reaction breaking a CC bond and forming a new alkylidene-titanium bond, the process then repeats itself with a second monomer:
Only much later the polynorbornene was going to be produced through ring opening metathesis polymerisation
. Giulio Natta
in 1964 also observed the formation of an unsaturated polymer when polymerizing cyclopentene
with tungsten and molybdenum halides .
In a third development leading up to olefin metathesis researchers at Phillips Petroleum Company in 1964 described olefin disproportionation
with catalysts molybdenum hexacarbonyl
, tungsten hexacarbonyl
, and molybdenum oxide supported on alumina for example converting propylene
to an equal mixture of ethylene
and 2-butene
for which they proposed a reaction mechanism
involving a cyclobutane
(they called it a quasicyclobutane) - metal complex:
This particular mechanism is symmetry forbidden based on the Woodward-Hoffmann rules
first formulated two years earlier. Cyclobutanes have also never been identified in metathesis reactions another reason why it was quickly abandoned.
Then in 1967 researchers at the Goodyear Tire and Rubber Company
described a novel catalyst system for the metathesis of 2-pentene based on tungsten hexachloride
, ethanol
the organoaluminum compound EtAlMe2 and also proposed a name for this reaction type: olefin metathesis . Formerly the reaction had been called "olefin disproportionation."
In this reaction 2-pentene forms a rapid (a matter of seconds) chemical equilibrium
with 2-butene
and 3-hexene. No double bond migrations are observed, the reaction can be started with the butene and hexene as well and the reaction can be stopped by addition of methanol
.
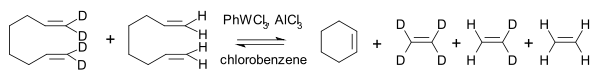
The Goodyear group demonstrated that the reaction of regular 2-butene with its all-deuterated isotopologue
yielded C4H4D4 with deuterium evenly distributed . In this way they were able to differentiate between a transalkylidenation mechanism and a transalkylation
mechanism (ruled out):
In 1971 Chauvin proposed a 4-membered metallocycle
intermediate to explain the statistical distribution of products found in certain metathesis reactions . This mechanism is today considered the actual mechanism taking place in olefin metathesis.
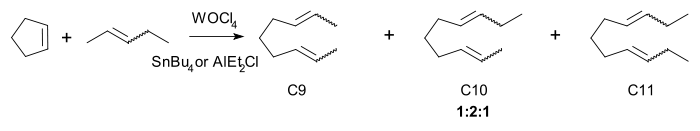
The active catalyst, a metallocarbene., was discovered by in 1964 by E. O. Fischer. Chauvins experimental evidence was based on the reaction of cyclopentene
and 2-pentene with the homogeneous catalyst tungsten(VI) oxytetrachloride
and tetrabutyltin
:
The three principal products C9, C10 and C11 are found in a 1:2:1 regardless of conversion. the same ratio is found with the higher oligomers. Chauvin also explained how the carbene forms in the first place: by alpha-hydride elimination from a carbon metal single bond. For example propylene
(C3) forms in a reaction of 2-butene (C4) with tungsten hexachloride
and tetramethyltin (C1).
In the same year Pettit who synthesised cyclobutadiene
a few years earlier independently came up with a competing mechanism . It consisted of a tetramethylene intermediate with sp3 hybridized carbon atoms linked to a central metal atom with multiple three-center two-electron bond
s.
Experimental support offered by Pettit for this mechanism was based on an observed reaction inhibition by carbon monoxide
in certain metathesis reactions of 4-nonene with a tungsten metal carbonyl
Robert H. Grubbs
got involved in metathesis in 1972 and also proposed a metallacycle intermediate but one with 4 carbon atoms in the ring . The group he worked in reacted 1,4-dilithiobutane with tungsten hexachloride in an attempt to directly produce a cyclomethylenemetallacycle producing an intermediate which yielded products identical with those produced by the intermediate in the olefin metathesis reaction. This mechanism is pairwise:
In 1973 Grubbs found further evidence for this mechanism by isolating one such metallacycle not with tungsten but with platinum
by reaction of the dilithiobutane with cis-bis(triphenylphosphine)dichloroplatinum(II)
In 1975 Katz also arrived at a metallacyclobutane intermediate consistent with the one proposed by Chauvin He reacted a mixture of cyclooctene
, 2-butene and 4-octene with a molybdenum
catalyst and observed that the unsymmetrical C14 hydrocarbon reaction product is present right from the start at low conversion.
In any of the pairwise mechanisms with olefin pairing as rate-determining step
this compound, a secondary reaction product of C12 with C6, would form well after formation of the two primary reaction products C12 and C16.
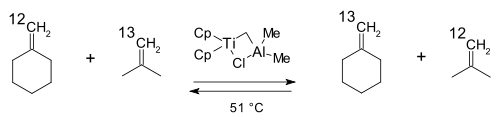
In 1974 Casey was the first to implement carbenes into the metathesis reaction mechanism:
Grubbs in 1976 provided evidence against his own updated pairwise mechanism:
with a 5-membered cycle in another round of isotope labeling studies in favor of the 4-membered cycle Chauvin mechanism:
In this reaction the ethylene
product distribution (d4,d2,d0) at low conversion was found to be consistent with the carbene mechanism. On the other hand Grubbs did not rule out the possibility of a tetramethylene intermediate.
The first practical metathesis system was introduced in 1978 by Tebbe based on the (what later became known as the) Tebbe reagent . In a model reaction isotopically labeled carbon atoms in isobutene and methylenecyclohexane
switched places:
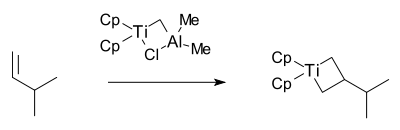
The Grubbs group then isolated the proposed metallacyclobutane intermediate in 1980 also with this reagent together with 3-methyl-1-butene:
They isolated a similar compound in the total synthesis
of capnellene
in 1986:
In that same year the Grubbs group proved that metathesis polymerization of norbornene by Tebbe's reagent is a living polymerization
system and a year later Grubbs and Schrock co-published an article describing living polymerization with a tungsten
carbene complex While Schrock focussed his research on tungsten and molybdenum catalysts for olefin metathesis, Grubbs started the development of catalysts based on ruthenium which proved to be less sensitive to oxygen and water and therefore more functional group
tolerant.
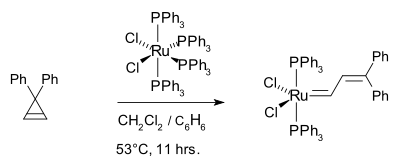
The corresponding tricyclohexylphosphine
complex (PCy3)2Cl2Ru=CHCH=CPh2 This work culminated in the now commercially available Grubbs catalyst.
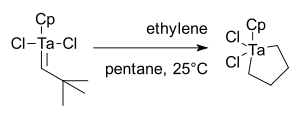
alkylidenes.. The initial result was disappointing as reaction of CpTa(=CH-t-Bu)Cl2 with ethylene
yielded only a metallacyclopentane, not metathesis products:
But by tweaking this structure to a PR3Ta(CHt-bu)(Ot-bu)2Cl (replacing chloride
by t-butoxide and a cyclopentadienyl
by an organophosphine, metathesis was established with cis-2-pentene. In another development, certain tungsten oxo complexes of the type W(O)(CHt-Bu)(Cl)2(PEt)3 were also found to be effective.
Schrock alkylidenes for olefin metathesis of the type Mo(NAr)(CHC(CH3)2R){OC(CH3)(CF3)2}2 were commercialized starting in 1990 .
The first asymmetric catalyst followed in 1993
with a Schrock catalyst modified with a BINOL ligand in a norbornadiene
ROMP
leading to highly stereoregular cis, isotactic polymer.
Organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, photochemical reactions and redox reactions. In organic synthesis,...
that entails redistribution of alkylene fragments by the scission of carbon - carbon double bonds
Chemical bond
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electromagnetic force attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction...
in olefins (alkenes). Its advantages include the creation of fewer sideproducts and hazardous waste
Hazardous waste
A hazardous waste is waste that poses substantial or potential threats to public health or the environment. According to the U.S. environmental laws hazardous wastes fall into two major categories: characteristic wastes and listed wastes.Characteristic hazardous wastes are materials that are known...
s. Yves Chauvin
Yves Chauvin
Yves Chauvin is a French chemist and Nobel Prize laureate. He is honorary research director at the Institut français du pétrole and a member of the French Academy of Science. Chauvin received his degree from the Lyon School of Chemistry, Physics and Electronics in 1954.He was awarded the 2005...
, Robert H. Grubbs
Robert H. Grubbs
Robert Howard Grubbs is an American chemist and Nobel laureate.As he noted in his official Nobel Prize autobiography, "In some places, my birthplace is listed as Calvert City and in others Possum Trot [NB: both in Marshall County]...
, and Richard R. Schrock
Richard R. Schrock
Richard Royce Schrock is an American chemist and Nobel laureate recognized for his contributions to the metathesis reaction used in organic chemistry.-Biography:...
shared the 2005 Nobel Prize in Chemistry
Nobel Prize in Chemistry
The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It is one of the five Nobel Prizes established by the will of Alfred Nobel in 1895, awarded for outstanding contributions in chemistry, physics, literature,...
for "the development of the metathesis method in organic synthesis
Organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the construction of organic compounds via organic reactions. Organic molecules can often contain a higher level of complexity compared to purely inorganic compounds, so the synthesis of organic compounds has...
".
The reaction is catalyzed
Catalysis
Catalysis is the change in rate of a chemical reaction due to the participation of a substance called a catalyst. Unlike other reagents that participate in the chemical reaction, a catalyst is not consumed by the reaction itself. A catalyst may participate in multiple chemical transformations....
by metals such as nickel
Nickel
Nickel is a chemical element with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile...
, tungsten
Tungsten
Tungsten , also known as wolfram , is a chemical element with the chemical symbol W and atomic number 74.A hard, rare metal under standard conditions when uncombined, tungsten is found naturally on Earth only in chemical compounds. It was identified as a new element in 1781, and first isolated as...
, rhenium
Rhenium
Rhenium is a chemical element with the symbol Re and atomic number 75. It is a silvery-white, heavy, third-row transition metal in group 7 of the periodic table. With an average concentration of 1 part per billion , rhenium is one of the rarest elements in the Earth's crust. The free element has...
, ruthenium
Ruthenium
Ruthenium is a chemical element with symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most chemicals. The Russian scientist Karl Ernst Claus discovered the element...
and molybdenum
Molybdenum
Molybdenum , is a Group 6 chemical element with the symbol Mo and atomic number 42. The name is from Neo-Latin Molybdaenum, from Ancient Greek , meaning lead, itself proposed as a loanword from Anatolian Luvian and Lydian languages, since its ores were confused with lead ores...
. The reaction consists of an alkene
Alkene
In organic chemistry, an alkene, olefin, or olefine is an unsaturated chemical compound containing at least one carbon-to-carbon double bond...
double bond
Double bond
A double bond in chemistry is a chemical bond between two chemical elements involving four bonding electrons instead of the usual two. The most common double bond, that between two carbon atoms, can be found in alkenes. Many types of double bonds between two different elements exist, for example in...
cleavage
Bond cleavage
Bond cleavage, or scission, is the splitting of chemical bonds.If the two electrons in a cleaved covalent bond are divided between the products, the process is known as homolytic fission and free redicals are generated by homolytic cleavage the process is known as homolytic fission or homolysis...
, followed by a statistical redistribution of alkylidene fragments. The general scope is outlined by the following scheme:

Applications
Olefin metathesis was first commercialized in petroleumPetroleum
Petroleum or crude oil is a naturally occurring, flammable liquid consisting of a complex mixture of hydrocarbons of various molecular weights and other liquid organic compounds, that are found in geologic formations beneath the Earth's surface. Petroleum is recovered mostly through oil drilling...
reformation for the synthesis of higher olefins from the products (α-olefins) from the Shell higher olefin process
Shell higher olefin process
The Shell higher olefin process is a chemical process for the production of linear alpha olefins via ethylene oligomerization and olefin metathesis invented and exploited by Royal Dutch Shell. The olefin products are converted to fatty aldehydes and then to fatty alcohols, which are precursors...
(SHOP) under high pressure and high temperatures. Traditional catalysts are derived from a reaction of the metal halides with alkylation agents for example WCl6-EtOH-EtAlCl2. A metathesis reaction is a chain reaction that begins when a metallocarbene and an olefin react to form a metallacyclobutane. This intermediate then reacts further, decomposing into a new olefin (the product) and a new metallocarbene, which can then be recycled through the reaction pathway.

Grubbs' catalyst
Grubbs' Catalyst is a transition metal carbene complex named after Robert H. Grubbs, the chemist who first synthesized it. There are two generations of the catalyst, as shown on the right. In contrast to other olefin metathesis catalysts, Grubbs' Catalysts tolerate other functional groups in the...
is a ruthenium carbenoid, whereas molybdenum(VI) and tungsten(VI)-based catalysts are known as Schrock alkylidene
Transition metal carbene complex
A transition metal carbene complex is a organometallic compound featuring a divalent organic ligand. The divalent organic ligand coordinated to the metal center is called a carbene. Carbene complexes for almost all transition metals have been reported. Many methods for synthesizing them and...
s. Related complexes can also catalyze alkyne metathesis
Alkyne metathesis
Alkyne metathesis is an organic reaction involving the redistribution of alkyne chemical bonds. This reaction is closely related to olefin metathesis. Metal-catalyzed alkyne metathesis was first described in 1968 by Bailey, et al. The Bailey system utilized a mixture of tungsten and silicon oxides...
and related polymerizations.
Reaction mechanism
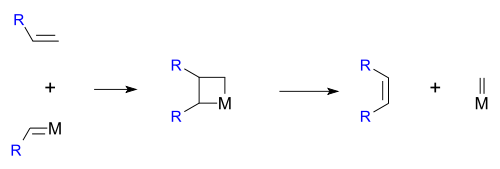
Hérisson and Chauvin first proposed the widely accepted mechanism of transition metal alkene metathesis. The direct [2+2] cycloaddition of two alkenes is formally symmetry forbiddenWoodward-Hoffmann rules
The Woodward–Hoffmann rules devised by Robert Burns Woodward and Roald Hoffmann are a set of rules in organic chemistry predicting the stereochemistry of pericyclic reactions based on orbital symmetry. These include electrocyclic reactions, cycloadditions , sigmatropic reactions, and group transfer...
and thus has a high activation energy
Activation energy
In chemistry, activation energy is a term introduced in 1889 by the Swedish scientist Svante Arrhenius that is defined as the energy that must be overcome in order for a chemical reaction to occur. Activation energy may also be defined as the minimum energy required to start a chemical reaction...
. The Chauvin mechanism involves the [2+2] cycloaddition of an alkene double bond to a transition metal alkylidene to form a metallocyclobutane intermediate. The metallocyclobutane produced can then cyclorevert to give either the original species or a new alkene and alkylidene. Interaction with the d-orbitals
Electron configuration
In atomic physics and quantum chemistry, electron configuration is the arrangement of electrons of an atom, a molecule, or other physical structure...
on the metal catalyst lowers the activation energy enough that the reaction can proceed rapidly at modest temperatures.
Metathesis chemistry
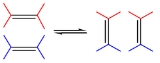
Some important classes of metathesisMetathesis (chemistry)
Salt metathesis is a molecular process involving the exchange of bonds between the two reacting chemical species, which results in the creation of products with similar or identical bonding affiliations. This is represented by the general reaction scheme:These chemical species can either be ionic...
chemistry:
- Alkane metathesisAlkane metathesisAlkane metathesis is a chemical reaction in which alkanes are rearranged to give longer or shorter alkane products. It is similar to olefin metathesis, except that olefin metathesis cleaves and recreates a carbon-carbon double bond, but alkane methathesis operates on a carbon-carbon single...
- Alkene metathesis
- Alkyne metathesisAlkyne metathesisAlkyne metathesis is an organic reaction involving the redistribution of alkyne chemical bonds. This reaction is closely related to olefin metathesis. Metal-catalyzed alkyne metathesis was first described in 1968 by Bailey, et al. The Bailey system utilized a mixture of tungsten and silicon oxides...
(AM) - Cross-metathesis (CM)
- Ring opening metathesis (ROM)
- Ring-closing metathesisRing-closing metathesisRing-closing metathesis or RCM is a variation on olefin metathesis that allows the closing of previously hard to make rings...
(RCM) - Enyne metathesisEnyne metathesisAn Enyne metathesis is an organic reaction taking place between an alkyne and an alkene with a metal carbene catalyst forming a butadiene. This reaction is a variation of olefin metathesis.The general scheme is given by scheme 1:...
(EM) - Ring opening metathesis polymerisationRing opening metathesis polymerisationRing-opening metathesis polymerization is a type of olefin metathesis chain-growth polymerization that produces industrially important products. The driving force of the reaction is relief of ring strain in cyclic olefins and a wide variety of catalysts have been discovered...
(ROMP) - Acyclic diene metathesisAcyclic diene metathesisAcyclic diene metathesis or ADMET is a special type of olefin metathesis used to polymerize certain terminal dienes to polyenes:The new double bonds formed can be in cis- or trans-configuation....
(ADMET)
Like most chemical reactions, the metathesis pathway is usually driven by a thermodynamic imperative; that is, the final products are determined by the energetics of the possible products, with a distribution of products proportional to the exponential of their respective energy values.
Alkene metathesis is often generally driven by the evolution of ethylene
Ethylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
, and alkyne metathesis is driven by the evolution of acetylene
Acetylene
Acetylene is the chemical compound with the formula C2H2. It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in pure form and thus is usually handled as a solution.As an alkyne, acetylene is unsaturated because...
, which are both gases; hence, metathesis reactions, if they are not ring-opening or ring-closing, usually use monosubstituted and 2,2-disubstituted alkenes and terminal alkynes. These are both dominated by the entropy gained by the net release of gas. Enyne
Enyne
An enyne is a functional group in organic chemistry consisting of a conjugated alkyne and alkene group....
metathesis cannot evolve a simple gas, and for that reason is usually disfavored unless there are accompanying ring-opening or ring-closing advantages. Ring opening metathesis usually involves a strained alkene (often a norbornene
Norbornene
Norbornene or norbornylene or norcamphene is a bridged cyclic hydrocarbon. It is a white solid with a pungent sour odor. The molecule consists of a cyclohexene ring bridged with a methylene group in the para position...
) and the release of ring strain drives the reaction. Ring-closing metathesis
Ring-closing metathesis
Ring-closing metathesis or RCM is a variation on olefin metathesis that allows the closing of previously hard to make rings...
, conversely, usually involves the formation of a five- or six-membered ring which is highly energetically favorable; although these reactions tend to also evolve ethylene
Ethylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
. RCM has been used to close larger macrocycles, in which case the reaction may be kinetically controlled by running the reaction at extreme dilutions. The Thorpe-Ingold effect
Thorpe-Ingold effect
The Thorpe–Ingold effect or gem-dimethyl effect, or angle compression is an effect observed in organic chemistry where increasing the size of two substituents on a tetrahedral center leads to enhanced reactions between parts of the other two substituents...
may be exploited to improve both reaction rates and selectivity.
Alkene metathesis is synthetically equivalent to (and has replaced) a procedure of ozonolysis
Ozonolysis
Ozonolysis is the cleavage of an alkene or alkyne with ozone to form organic compounds in which the multiple carbon–carbon bond has been replaced by a double bond to oxygen...
of an alkene to two ketone fragments followed by the reaction of one of them with a Wittig reagent.
Scope
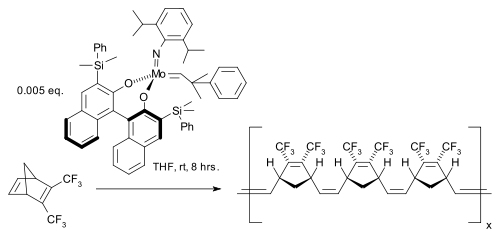
One study reported a ring-opening cross-olefin metathesis based on a Hoveyda-Grubbs Catalyst:
1-Hexene
1-Hexene is an organic compound with the formula CH2CHC4H9. It is an alkene that is classified in industry as higher olefin and an alpha-olefin, the latter term meaning that the double bond is located at the alpha position, endowing the compound with higher reactivity and thus useful chemical...
with the WCl4(OAr)2 catalyst yields 5-decene plus many byproducts from secondary metathesis reactions.
Historical overview
Known chemistry prior to the advent of olefin metathesis was introduced by Karl ZieglerKarl Ziegler
Karl Waldemar Ziegler was a German chemist who won the Nobel Prize in Chemistry in 1963, with Giulio Natta, for work on polymers. The Nobel Committee recognized his "excellent work on organometallic compounds [which]...led to new polymerization reactions and ... paved the way for new and highly...
in the 1950s who as part of ongoing work in what would later become known as Ziegler-Natta catalysis studied ethylene
Ethylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
polymerization
Polymerization
In polymer chemistry, polymerization is a process of reacting monomer molecules together in a chemical reaction to form three-dimensional networks or polymer chains...
which on addition of certain metals resulted in 1-butene
1-Butene
1-Butene is an organic chemical compound, linear alpha-olefin , and one of the isomers of butene. The formula is .-Stability:1-Butene is stable in itself but polymerizes exothermically. It is highly flammable and readily forms explosive mixtures with air...
instead of a saturated long-chain hydrocarbon (see nickel effect) .
In 1960 a Du Pont research group polymerized norbornene
Norbornene
Norbornene or norbornylene or norcamphene is a bridged cyclic hydrocarbon. It is a white solid with a pungent sour odor. The molecule consists of a cyclohexene ring bridged with a methylene group in the para position...
to polynorbornene using lithium aluminum tetraheptyl and titanium tetrachloride
Titanium tetrachloride
Titanium tetrachloride is the inorganic compound with the formula TiCl4. It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. TiCl4 is an unusual example of a metal halide that is highly volatile...
(a patent by this company on this topic dates back to 1955 ),
a reaction then classified as a so-called coordination polymerization
Coordination polymerization
Coordination polymerization is a form of addition polymerization in which monomer adds to a growing macromolecule through an organometallic active center...
. According to the then proposed reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
a RTiX titanium intermediate first coordinates to the double bond in a pi complex. The second step then is a concerted SNi reaction breaking a CC bond and forming a new alkylidene-titanium bond, the process then repeats itself with a second monomer:
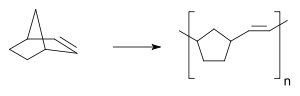
Only much later the polynorbornene was going to be produced through ring opening metathesis polymerisation
Ring opening metathesis polymerisation
Ring-opening metathesis polymerization is a type of olefin metathesis chain-growth polymerization that produces industrially important products. The driving force of the reaction is relief of ring strain in cyclic olefins and a wide variety of catalysts have been discovered...
. Giulio Natta
Giulio Natta
Giulio Natta was an Italian chemist and Nobel laureate. He won a Nobel Prize in Chemistry in 1963 with Karl Ziegler for work on high polymers.-Early years:...
in 1964 also observed the formation of an unsaturated polymer when polymerizing cyclopentene
Cyclopentene
Cyclopentene is a chemical compound with the formula 58. It is a colorless liquid with a petrol-like odor. It is one of the cycloalkenes.Cyclopentene is produced industrially in large amounts...
with tungsten and molybdenum halides .
In a third development leading up to olefin metathesis researchers at Phillips Petroleum Company in 1964 described olefin disproportionation
Disproportionation
Disproportionation, also known as dismutation is used to describe a specific type of redox reaction in which a species is simultaneously reduced and oxidized so as to form two different products....
with catalysts molybdenum hexacarbonyl
Molybdenum hexacarbonyl
Molybdenum hexacarbonyl is the chemical compound with the formula Mo6. This colorless solid, like its chromium and tungsten analogues, is noteworthy as a volatile, air-stable derivative of a metal in its zero oxidation state.-Structure and properties:Mo6 adopts an octahedral geometry consisting...
, tungsten hexacarbonyl
Tungsten hexacarbonyl
Tungsten hexacarbonyl is the chemical compound with the formula W6. This complex gave rise to the first example of a dihydrogen complex....
, and molybdenum oxide supported on alumina for example converting propylene
Propylene
Propene, also known as propylene or methylethylene, is an unsaturated organic compound having the chemical formula C3H6. It has one double bond, and is the second simplest member of the alkene class of hydrocarbons, and it is also second in natural abundance.-Properties:At room temperature and...
to an equal mixture of ethylene
Ethylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
and 2-butene
2-Butene
2-Butene is an acyclic alkene with four carbon atoms. It is the simplest alkene exhibiting cis/trans-isomerism ; that is, it exists as two geometrical isomers cis-2-butene , shown at the right, and trans-2-butene , not shown.It is a petrochemical, produced by the catalytic cracking of crude oil...
for which they proposed a reaction mechanism
Reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs.Although only the net chemical change is directly observable for most chemical reactions, experiments can often be designed that suggest the possible sequence of steps in...
involving a cyclobutane
Cyclobutane
Cyclobutane is an organic compound with the formula 4. Cyclobutane is a colourless gas and commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes...
(they called it a quasicyclobutane) - metal complex:
This particular mechanism is symmetry forbidden based on the Woodward-Hoffmann rules
Woodward-Hoffmann rules
The Woodward–Hoffmann rules devised by Robert Burns Woodward and Roald Hoffmann are a set of rules in organic chemistry predicting the stereochemistry of pericyclic reactions based on orbital symmetry. These include electrocyclic reactions, cycloadditions , sigmatropic reactions, and group transfer...
first formulated two years earlier. Cyclobutanes have also never been identified in metathesis reactions another reason why it was quickly abandoned.
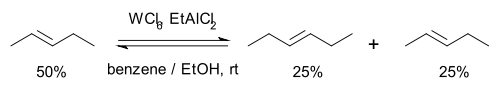
Then in 1967 researchers at the Goodyear Tire and Rubber Company
Goodyear Tire and Rubber Company
The Goodyear Tire & Rubber Company was founded in 1898 by Frank Seiberling. Goodyear manufactures tires for automobiles, commercial trucks, light trucks, SUVs, race cars, airplanes, farm equipment and heavy earth-mover machinery....
described a novel catalyst system for the metathesis of 2-pentene based on tungsten hexachloride
Tungsten hexachloride
Tungsten hexachloride is the chemical compound of tungsten and chlorine with the formula WCl6. This dark violet blue species exists as a volatile solid under standard conditions. It is an important starting reagent in the preparation of tungsten compounds. WCl6 is a rare example of a...
, ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
the organoaluminum compound EtAlMe2 and also proposed a name for this reaction type: olefin metathesis . Formerly the reaction had been called "olefin disproportionation."
In this reaction 2-pentene forms a rapid (a matter of seconds) chemical equilibrium
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
with 2-butene
2-Butene
2-Butene is an acyclic alkene with four carbon atoms. It is the simplest alkene exhibiting cis/trans-isomerism ; that is, it exists as two geometrical isomers cis-2-butene , shown at the right, and trans-2-butene , not shown.It is a petrochemical, produced by the catalytic cracking of crude oil...
and 3-hexene. No double bond migrations are observed, the reaction can be started with the butene and hexene as well and the reaction can be stopped by addition of methanol
Methanol
Methanol, also known as methyl alcohol, wood alcohol, wood naphtha or wood spirits, is a chemical with the formula CH3OH . It is the simplest alcohol, and is a light, volatile, colorless, flammable liquid with a distinctive odor very similar to, but slightly sweeter than, ethanol...
.
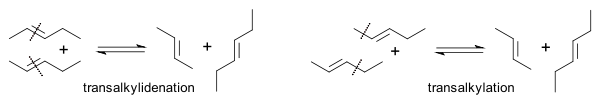
The Goodyear group demonstrated that the reaction of regular 2-butene with its all-deuterated isotopologue
Isotopologue
Isotopologues are molecules that differ only in their isotopic composition. Simply, the isotopologue of a chemical species has at least one atom with a different number of neutrons than the parent....
yielded C4H4D4 with deuterium evenly distributed . In this way they were able to differentiate between a transalkylidenation mechanism and a transalkylation
Transalkylation
Transalkylation is a chemical reaction of transfer of an alkyl group from one organic compound to another. Zeolite catalysts are often used. The reaction is widely used in the petrochemical industry to manufacture p-xylene, styrene, and other aromatic compounds....
mechanism (ruled out):
In 1971 Chauvin proposed a 4-membered metallocycle
Metallocycle
In organometallic chemistry, a metallacycle is a derivative of a carbocyclic compound wherein a metal has replaced at least one carbon center. Metallacycles appear frequently as reactive intermediates in catalysis, e.g. olefin metathesis and alkyne trimerization. In organic synthesis, directed...
intermediate to explain the statistical distribution of products found in certain metathesis reactions . This mechanism is today considered the actual mechanism taking place in olefin metathesis.
The active catalyst, a metallocarbene., was discovered by in 1964 by E. O. Fischer. Chauvins experimental evidence was based on the reaction of cyclopentene
Cyclopentene
Cyclopentene is a chemical compound with the formula 58. It is a colorless liquid with a petrol-like odor. It is one of the cycloalkenes.Cyclopentene is produced industrially in large amounts...
and 2-pentene with the homogeneous catalyst tungsten(VI) oxytetrachloride
Tungsten(VI) oxytetrachloride
Tungsten oxytetrachloride,also known as Tungsten tetrachloride oxide, is an inorganic compound with the formula WOCl4. This diamagnetic solid is used to prepare other complexes of tungsten. The orange-coloured compound is soluble in nonpolar solvents but it reacts with alcohols and water and...
and tetrabutyltin
Tetrabutyltin
Tetrabutyltin is a stable organotin compound and combustible, colourless liquid at room temperature. It is incompatible with strong oxidizing agents. It has the molecular formula C16H36Sn. Sometimes the abbreviation SnBu4 and TTBT are used.Tetrabutyltin is the starting material of the tributyltin...
:
The three principal products C9, C10 and C11 are found in a 1:2:1 regardless of conversion. the same ratio is found with the higher oligomers. Chauvin also explained how the carbene forms in the first place: by alpha-hydride elimination from a carbon metal single bond. For example propylene
Propylene
Propene, also known as propylene or methylethylene, is an unsaturated organic compound having the chemical formula C3H6. It has one double bond, and is the second simplest member of the alkene class of hydrocarbons, and it is also second in natural abundance.-Properties:At room temperature and...
(C3) forms in a reaction of 2-butene (C4) with tungsten hexachloride
Tungsten hexachloride
Tungsten hexachloride is the chemical compound of tungsten and chlorine with the formula WCl6. This dark violet blue species exists as a volatile solid under standard conditions. It is an important starting reagent in the preparation of tungsten compounds. WCl6 is a rare example of a...
and tetramethyltin (C1).
In the same year Pettit who synthesised cyclobutadiene
Cyclobutadiene
Cyclobutadiene is the smallest [n]-annulene , an extremely unstable hydrocarbon having a lifetime shorter than five seconds in the free state. It has chemical formula 44 and a rectangular structure verified by infrared studies. This is in contrast to the square geometry predicted by simple Hückel...
a few years earlier independently came up with a competing mechanism . It consisted of a tetramethylene intermediate with sp3 hybridized carbon atoms linked to a central metal atom with multiple three-center two-electron bond
Three-center two-electron bond
A three-center two-electron bond is an electron-deficient chemical bond where three atoms share two electrons. The combination of three atomic orbitals form three molecular orbitals: one bonding, one non-bonding, and one anti-bonding. The two electrons go into the bonding orbital, resulting in a...
s.
Experimental support offered by Pettit for this mechanism was based on an observed reaction inhibition by carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
in certain metathesis reactions of 4-nonene with a tungsten metal carbonyl
Metal carbonyl
Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. These complexes may be homoleptic, that is containing only CO ligands, such as nickel carbonyl , but more commonly metal carbonyls contain a mix of ligands, such as Re3Cl...
Robert H. Grubbs
Robert H. Grubbs
Robert Howard Grubbs is an American chemist and Nobel laureate.As he noted in his official Nobel Prize autobiography, "In some places, my birthplace is listed as Calvert City and in others Possum Trot [NB: both in Marshall County]...
got involved in metathesis in 1972 and also proposed a metallacycle intermediate but one with 4 carbon atoms in the ring . The group he worked in reacted 1,4-dilithiobutane with tungsten hexachloride in an attempt to directly produce a cyclomethylenemetallacycle producing an intermediate which yielded products identical with those produced by the intermediate in the olefin metathesis reaction. This mechanism is pairwise:
In 1973 Grubbs found further evidence for this mechanism by isolating one such metallacycle not with tungsten but with platinum
Platinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
by reaction of the dilithiobutane with cis-bis(triphenylphosphine)dichloroplatinum(II)
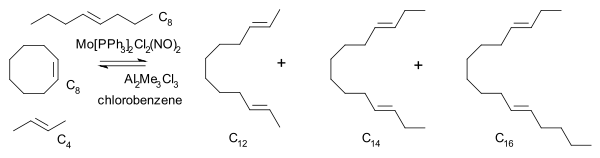
In 1975 Katz also arrived at a metallacyclobutane intermediate consistent with the one proposed by Chauvin He reacted a mixture of cyclooctene
Cyclooctene
Cyclooctene is a cycloalkene with an eight-membered ring. It is notable because it is the smallest cycloalkene that can exist as either the cis- or trans-isomer with the cis-isomer more common...
, 2-butene and 4-octene with a molybdenum
Molybdenum
Molybdenum , is a Group 6 chemical element with the symbol Mo and atomic number 42. The name is from Neo-Latin Molybdaenum, from Ancient Greek , meaning lead, itself proposed as a loanword from Anatolian Luvian and Lydian languages, since its ores were confused with lead ores...
catalyst and observed that the unsymmetrical C14 hydrocarbon reaction product is present right from the start at low conversion.
In any of the pairwise mechanisms with olefin pairing as rate-determining step
Rate-determining step
The rate-determining step is a chemistry term for the slowest step in a chemical reaction. The rate-determining step is often compared to the neck of a funnel; the rate at which water flows through the funnel is determined by the width of the neck, not by the speed at which water is poured in. In...
this compound, a secondary reaction product of C12 with C6, would form well after formation of the two primary reaction products C12 and C16.
In 1974 Casey was the first to implement carbenes into the metathesis reaction mechanism:
Grubbs in 1976 provided evidence against his own updated pairwise mechanism:
with a 5-membered cycle in another round of isotope labeling studies in favor of the 4-membered cycle Chauvin mechanism:
In this reaction the ethylene
Ethylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
product distribution (d4,d2,d0) at low conversion was found to be consistent with the carbene mechanism. On the other hand Grubbs did not rule out the possibility of a tetramethylene intermediate.
The first practical metathesis system was introduced in 1978 by Tebbe based on the (what later became known as the) Tebbe reagent . In a model reaction isotopically labeled carbon atoms in isobutene and methylenecyclohexane
Methylenecyclohexane
Methylenecyclohexane is a very useful compound in organic syntheses. It can be produced by a Wittig reaction or a Tebbe reaction from cyclohexanone, or as a side product of the dehydration of 2-methylcyclohexanol into 1-methylcyclohexene....
switched places:
The Grubbs group then isolated the proposed metallacyclobutane intermediate in 1980 also with this reagent together with 3-methyl-1-butene:
They isolated a similar compound in the total synthesis
Total synthesis
In organic chemistry, a total synthesis is, in principle, the complete chemical synthesis of complex organic molecules from simpler pieces, usually without the aid of biological processes. In practice, these simpler pieces are commercially available in bulk and semi-bulk quantities, and are often...
of capnellene
Capnellene
Capnellene is a naturally occurring hydrocarbon derived from Capnella imbricata, a species of soft coral found in Indonesia. Since the 1970s, capnellene has been targeted for synthesis by numerous investigators due to its stereochemistry, functionality, and the interesting geometry of the carbon...
in 1986:
In that same year the Grubbs group proved that metathesis polymerization of norbornene by Tebbe's reagent is a living polymerization
Living polymerization
In polymer chemistry, living polymerization is a form of addition polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer reactions are absent and the rate of chain initiation is...
system and a year later Grubbs and Schrock co-published an article describing living polymerization with a tungsten
Tungsten
Tungsten , also known as wolfram , is a chemical element with the chemical symbol W and atomic number 74.A hard, rare metal under standard conditions when uncombined, tungsten is found naturally on Earth only in chemical compounds. It was identified as a new element in 1781, and first isolated as...
carbene complex While Schrock focussed his research on tungsten and molybdenum catalysts for olefin metathesis, Grubbs started the development of catalysts based on ruthenium which proved to be less sensitive to oxygen and water and therefore more functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
tolerant.
Grubbs catalysts
In earlier work, Michelotti and Keaveney demonstrated that the ring-opening polymerization of norbornene was catalyzed by hydrated trichlorides of ruthenium, osmium, and iridium in alcoholic solvents, Francis W. Michelotti and William P. Keaveney, J. Polymer Science: Part A, Vol. 3, PP 895-905 (1965), Coordinated Polymerization of the Bicyclo-(2.2.1)-heptene-2 Ring System (Norbornene) in Polar Media. In 1965, Rinehart and Smith demonstrated that norbornene can be polymerized in emulsion using an iridium or ruthenium halide catalyst, Robert E. Rinehart and Homer P. Smith, Polymer Letters Vol. 3, PP.1049-1052 (1965). In 1974, Lido Porri et al. reported on the Ring-Opening Polymerization of Cycloolefins with Catalysts Derived from Ruthenium and Iridium, Die Makromolekulare Chemie, 175, 3097-3115 (1974). They conclude that the reaction of norbornene and aliphatic olefins proceed by a metathesis mechanism. This theme was picked up years later by the Grubbs group, who polymerized the 7-oxo norbornene derivative using ruthenium trichloride, osmium trichloride as well as tungsten alkylidenes.. They identified a Ru(II) carbene as an effective metal center such as (PPh3)2Cl2Ru=CHCH=CPh2:The corresponding tricyclohexylphosphine
Tricyclohexylphosphine
Tricyclohexylphosphine is the tertiary phosphine with the formula P3. Commonly used as a ligand in organometallic chemistry, it is often abbreviated to PCy3, where Cy stands for cyclohexyl...
complex (PCy3)2Cl2Ru=CHCH=CPh2 This work culminated in the now commercially available Grubbs catalyst.
Schrock catalysts
Schrock entered the olefin metathesis field in 1979 as an extension of work on tantalumTantalum
Tantalum is a chemical element with the symbol Ta and atomic number 73. Previously known as tantalium, the name comes from Tantalus, a character in Greek mythology. Tantalum is a rare, hard, blue-gray, lustrous transition metal that is highly corrosion resistant. It is part of the refractory...
alkylidenes.. The initial result was disappointing as reaction of CpTa(=CH-t-Bu)Cl2 with ethylene
Ethylene
Ethylene is a gaseous organic compound with the formula . It is the simplest alkene . Because it contains a carbon-carbon double bond, ethylene is classified as an unsaturated hydrocarbon. Ethylene is widely used in industry and is also a plant hormone...
yielded only a metallacyclopentane, not metathesis products:
But by tweaking this structure to a PR3Ta(CHt-bu)(Ot-bu)2Cl (replacing chloride
Chloride
The chloride ion is formed when the element chlorine, a halogen, picks up one electron to form an anion Cl−. The salts of hydrochloric acid HCl contain chloride ions and can also be called chlorides. The chloride ion, and its salts such as sodium chloride, are very soluble in water...
by t-butoxide and a cyclopentadienyl
Cyclopentadienyl
In organic chemistry, cyclopentadienyl is a cyclic group of atoms with the formula C5H5. Cyclopentadienyl are closely related to cyclopentadiene. Cyclopentadienyl have five carbon atoms bonded together in a pentagonal planar ring, all five of which are bonded to individual hydrogen atoms...
by an organophosphine, metathesis was established with cis-2-pentene. In another development, certain tungsten oxo complexes of the type W(O)(CHt-Bu)(Cl)2(PEt)3 were also found to be effective.
Schrock alkylidenes for olefin metathesis of the type Mo(NAr)(CHC(CH3)2R){OC(CH3)(CF3)2}2 were commercialized starting in 1990 .
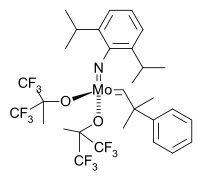
The first asymmetric catalyst followed in 1993
with a Schrock catalyst modified with a BINOL ligand in a norbornadiene
Norbornadiene
Norbornadiene is an organic compound. This bicyclic hydrocarbon is the most stable diolefin derived from the norbornane and norbornene. Norbornadiene is primarily of interest as a ligand in homogeneous catalysis, but it has been heavily studied due to its high reactivity and distinctive...
ROMP
Ring opening metathesis polymerisation
Ring-opening metathesis polymerization is a type of olefin metathesis chain-growth polymerization that produces industrially important products. The driving force of the reaction is relief of ring strain in cyclic olefins and a wide variety of catalysts have been discovered...
leading to highly stereoregular cis, isotactic polymer.