
Nickel
Encyclopedia
Nickel is a chemical element
with the chemical symbol
Ni and atomic number
28. It is a silvery-white lustrous metal
with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile
. Pure nickel shows a significant chemical activity, though larger pieces of the metal are slow to react with air at ambient conditions due to the formation of a protective oxide surface. However, nickel is reactive with oxygen to the extent that native
nickel is rare on Earth's surface, and is mostly confined to the interiors of larger nickel iron meteorites, which were protected from oxidation in space. Such native nickel is always found on Earth alloyed with iron, in keeping with the element's origin as a major end-product of the nucleosynthesis
process, along with iron, in supernovas. An iron-nickel alloy is thought to compose the Earth's core
.
The use of nickel (as a natural meteoric nickel-iron alloy) has been traced as far back as 3500 BC. Nickel was first isolated and classified as a chemical element in 1751 by Axel Fredrik Cronstedt
, who initially mistook its ore
for a copper mineral. Its most important ore minerals are laterite
s, including limonite
, garnierite
, and pentlandite
. Major production sites include Sudbury region
in Canada (which is thought to be of meteoric origin), New Caledonia
and Norilsk
in Russia.
Because of nickel's slow rate of oxidation at room temperature, it is considered corrosion-resistant. Historically this has led to its use for plating metals such as iron and brass
, to its use for chemical apparatus, and its use in certain alloys that will retain a high silvery polish, such as German silver
. About 6% of world nickel production is still used for corrosion-resistant pure-nickel plating. Nickel was once a common component of coins, but has largely been replaced by cheaper iron for this purpose, especially since the metal has proven to be a skin allergen
for some people.
Nickel is one of the four elements that are ferromagnetic around room temperature. Alnico
permanent magnets based partly on nickel are of intermediate strength between iron-based permanent magnets and rare earth magnets
. The metal is chiefly valuable in the modern world for the alloy
s it forms; about 60% of world production is used in nickel-steels (particularly stainless steel
). Other common alloys, as well as some new superalloy
s, make up most of the remainder of world nickel use, with chemical uses for nickel compounds consuming less than 3% of production. As a compound, nickel has a number of niche chemical manufacturing uses, such as a catalyst for hydrogenation
. Enzymes of some microorganisms and plants contain nickel as an active center, which makes the metal an essential nutrient for them.
and gadolinium
. Its Curie temperature is 355 °C, meaning that bulk nickel is non-magnetic above this temperature. The unit cell of nickel is a face centered cube
with the lattice parameter of 0.352 nm giving an atomic radius
of 0.124 nm. Nickel belongs to the transition metals and is hard and ductile
.
The nickel atom has two electron configuration
s, [Ar] 4s2 3d8 and [Ar] 4s1 3d9, which are very close in energy, where the symbol [Ar] refers to the argon
-like core structure. There is some disagreement as to which should be considered the lowest energy configuration. Chemistry textbooks quote the electron configuration of nickel as [Ar] 4s2 3d8, or equivalently as [Ar] 3d8 4s2. This configuration agrees with the Madelung energy ordering rule, which predicts that 4s is filled before 3d. It is supported by the experimental fact that the lowest energy state of the nickel atom is a 4s2 3d8 energy level, specifically the 3d8(3F) 4s2 3F, J=4 level.
However each of these two configurations in fact gives rise to a set of states at different energies. The two sets of energies overlap, and the average energy of states having configuration [Ar] 4s1 3d9 is in fact lower than the average energy of states having configuration [Ar] 4s2 3d8. For this reason the research literature on atomic calculations quotes the ground state configuration of nickel as 4s1 3d9.
s; 58Ni, 60Ni, 61Ni, 62Ni and 64Ni with 58Ni being the most abundant (68.077% natural abundance
). 62Ni
is the "most stable" nuclide of all the existing elements, with binding energy greater than both 56Fe
, often incorrectly cited as "most stable", and 58Fe. 18 radioisotopes have been characterised with the most stable being 59Ni with a half-life
of 76,000 years, 63Ni with a half-life of 100.1 years, and 56Ni with a half-life of 6.077 days. All of the remaining radioactive isotopes have half-lives that are less than 60 hours and the majority of these have half-lives that are less than 30 seconds. This element also has 1 meta state.
Nickel-56 is produced by the silicon burning process
and later set free in large quantities during type Ia supernova
e. Indeed, the shape of the light curve
of these supernovae at intermediate to late-times corresponds to the decay via electron capture
of nickel-56 to cobalt
-56 and ultimately to iron-56. Nickel-59 is a long-lived cosmogenic
radionuclide
with a half-life of 76,000 years. 59Ni has found many applications in isotope geology. 59Ni has been used to date the terrestrial age of meteorite
s and to determine abundances of extraterrestrial dust in ice and sediment
. Nickel-60 is the daughter product of the extinct radionuclide 60Fe, which decays with a half-life of 2.6 million years. Because 60Fe has such a long half-life, its persistence in materials in the solar system
at high enough concentrations may have generated observable variations in the isotopic composition of 60Ni. Therefore, the abundance of 60Ni present in extraterrestrial material may provide insight into the origin of the solar system and its early history. Nickel-62
has the highest binding energy
per nucleon of any isotope for any element (8.7946 Mev/nucleon). Isotopes heavier than 62Ni cannot be formed by nuclear fusion
without losing energy. Nickel-48, discovered in 1999, is the most proton-rich heavy element isotope known. With 28 proton
s and 20 neutron
s 48Ni is "double magic" (like 208Pb
) and therefore unusually stable.
The isotopes of nickel range in atomic weight
from 48 u
to 78 u . Nickel-78's half-life was recently measured to be 110 milliseconds and is believed to be an important isotope involved in supernova nucleosynthesis
of elements heavier than iron.
and iron in pentlandite
, with sulfur
in millerite
, with arsenic
in the mineral nickeline, and with arsenic and sulfur
in nickel galena
. Nickel is commonly found in iron meteorite
s as the alloys kamacite
and taenite
.
The bulk of the nickel mined comes from two types of ore
deposits. The first are laterite
s where the principal ore minerals are nickeliferous limonite
: (Fe, Ni)O(OH) and garnierite
(a hydrous nickel silicate): (Ni, Mg)3Si2O5(OH)4. The second are magmatic sulfide deposits where the principal ore mineral is pentlandite
: (Ni, Fe)9S8.
In terms of supply, the Sudbury region of Ontario
, Canada, produces about 30% of the world's supply of nickel. The Sudbury Basin deposit is theorized to have been created by a meteorite
impact event
early in the geologic history of Earth
. Russia contains about 40% of the world's known resources at the Norilsk deposit in Siberia
. The Russian mining company MMC Norilsk Nickel
obtains the nickel and the associated palladium
for world distribution. Other major deposits of nickel are found in New Caledonia
, France, Australia, Cuba, and Indonesia. Deposits found in tropical areas typically consist of laterites, which are produced by the intense weathering of ultramafic igneous rocks and the resulting secondary concentration of nickel bearing oxide and silicate minerals
.
Based on geophysical
evidence, most of the nickel on Earth is postulated to be concentrated in the Earth's core. Kamacite
and taenite
are naturally occurring alloy
s of iron and nickel. For kamacite the alloy is usually in the proportion of 90:10 to 95:5 although impurities such as cobalt
or carbon
may be present, while for taenite the nickel content is between 20% and 65%. Kamacite and taenite occur in nickel iron meteorites.
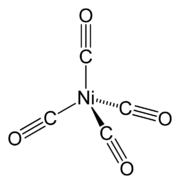 The most common oxidation state
The most common oxidation state
of nickel is +2, but compounds of Ni0, Ni+, and Ni3+ are well known, and Ni4+ has been demonstrated.
, is a volatile liquid at room temperature. On heating, the complex decomposes back to nickel and carbon monoxide:
This behavior is exploited in the Mond process
for purifying nickel, as described above. The related nickel(0) complex bis(cyclooctadiene)nickel(0)
is a useful catalyst in organonickel chemistry due to the easily displaced cod ligands.
-sulfate-hexahydrate-sample.jpg) Nickel(II) compounds are known with all common anions, i.e. the sulfide, sulfate, carbonate, hydroxide, carboxylates, and halides. Nickel(II) sulfate
Nickel(II) compounds are known with all common anions, i.e. the sulfide, sulfate, carbonate, hydroxide, carboxylates, and halides. Nickel(II) sulfate
is produced in large quantities by dissolving nickel metal or oxides in sulfuric acid. It exists as both a hexa- and heptahydrates. This compound is useful for electroplating
nickel.
The four halogens form nickel compounds, all of which adopt octahedral geometries. Nickel(II) chloride
is most common, and its behavior is illustrative of the other halides. Nickel(II) chloride is produced by dissolving nickel residues in hydrochloric acid. The dichloride is usually encountered as the green hexahydrate, but it can be dehydrated to give the yellow anhydrous NiCl2. Some tetracoordinate nickel(II) complexes form both tetrahedral and square planar geometries. The tetrahedral complexes are paramagnetic and the square planar complexes are diamagnetic. This equilibrium as well as the formation of octahedral complexes contrasts with the behavior of the divalent complexes of the heavier group 10 metals, palladium(II) and platinum(II), which tend to adopt only square-planar complexes.
Nickelocene
is known; it has an electron count of 20, making it relatively unstable.
is used as the cathode
in many rechargeable batteries, including nickel-cadmium, nickel-iron
, nickel hydrogen
, and nickel-metal hydride, and used by certain manufacturers in Li-ion batteries.
s from what is now Syria had contained up to 2% nickel. Further, there are Chinese manuscripts suggesting that "white copper" (cupronickel
, known as baitung) was used there between 1700 and 1400 BC. This Paktong white copper was exported to Britain as early as the 17th century, but the nickel content of this alloy was not discovered until 1822.
In medieval Germany, a red mineral was found in the Erzgebirge (Ore Mountains) that resembled copper ore. However, when miners were unable to extract any copper from it, they blamed a mischievous sprite of German mythology, Nickel (similar to Old Nick), for besetting the copper. They called this ore Kupfernickel from the German Kupfer for copper. This ore is now known to be nickeline or niccolite
, a nickel arsenide
. In 1751, Baron Axel Fredrik Cronstedt
was attempting to extract copper from kupfernickel and obtained instead a white metal that he named after the spirit which had given its name to the mineral, nickel. In modern German, Kupfernickel or Kupfer-Nickel designates the alloy cupronickel
.
After its discovery, the only source for nickel was the rare Kupfernickel, but, from 1824 on, the nickel was obtained as byproduct of cobalt blue
production. The first large-scale producer of nickel was Norway, which exploited nickel-rich pyrrhotite
from 1848 on. The introduction of nickel in steel production in 1889 increased the demand for nickel, and the nickel deposits of New Caledonia
, which were discovered in 1865, provided most of the world's supply between 1875 and 1915. The discovery of the large deposits in the Sudbury Basin, Canada in 1883, in Norilsk-Talnakh
, Russia in 1920, and in the Merensky Reef
, South Africa in 1924 made large-scale production of nickel possible.
Nickel has been a component of coins since the mid-19th century. In the United States, the term "nickel" or "nick" was originally applied to the copper-nickel Flying Eagle cent
, which replaced copper with 12% nickel 1857–58, then the Indian Head cent
of the same alloy from 1859–1864. Still later in 1865, the term designated the three-cent nickel
, with nickel increased to 25%. In 1866, the five-cent shield nickel
(25% nickel, 75% copper) appropriated the designation. Along with the alloy proportion, this term has been used to the present in the United States. Coins of nearly pure nickel were first used in 1881 in Switzerland, and more notably 99.9% nickel five-cent coins
were struck in Canada (the world's largest nickel producer at the time) during non-war years from 1922–1981, and their metal content made these coins magnetic. During the wartime period 1942–45, more or all nickel was removed from Canadian and U.S. coins, due to nickel's war-critical use in armor. Canada switched alloys again to plated steel during the Korean war
, but was forced to stop making pure nickel "nickels" in 1981, reserving the pure 99.9% nickel alloy after 1968 only to its higher-value coins. Finally, in the 21st century, with rising nickel prices, most countries that formerly used nickel in their coins have abandoned the metal for cost reasons, and the U.S. five-cent coin remains one of the few in which the metal is still used, save for exterior plating.
2.png) In 2005, Russia was the largest producer of nickel with about one-fifth world share closely followed by Canada, Australia, and Indonesia, as reported by the British Geological Survey
In 2005, Russia was the largest producer of nickel with about one-fifth world share closely followed by Canada, Australia, and Indonesia, as reported by the British Geological Survey
. A nickel deposit in western Turkey had been exploited, with this location being especially convenient for European smelters, steelmakers, and factories. The one locality in the United States where nickel was commercially mined is Riddle, Oregon
, where several square miles of nickel-bearing garnierite surface deposits are located. The mine closed in 1987. The Eagle mine project
is a proposed new nickel mine in Michigan
's upper peninsula
.
. Nickel is extracted from its ores by conventional roasting and reduction processes that yield a metal of greater than 75% purity. In many stainless steel
applications, 75% pure nickel can be used without further purification, depending on the composition of the impurities.
Most sulfide ores have traditionally been processed using pyrometallurgical techniques to produce a matte
for further refining. Recent advances in hydrometallurgy
have resulted in significant nickel purification using these processes. Most sulfide deposits have traditionally been processed by concentration through a froth flotation
process followed by pyrometallurgical extraction. In hydrometallurgical processes, nickel sulfide ores undergo flotation (differential flotation if Ni/Fe ratio is too low) and then smelted. After producing the nickel matte, further processing is done via the Sherritt-Gordon process. First, copper is removed by adding hydrogen sulfide
, leaving a concentrate of only cobalt and nickel. Then, solvent extraction is used to separate the cobalt and nickel, with the final nickel concentration greater than 99%.
, which increases the nickel concentrate to greater than 99.99% purity. This process was patented by L. Mond and has been in industrial use since before the beginning of the 20th century. In the process, nickel is reacted with carbon monoxide
at around 40–80 °C to form nickel carbonyl
in the presence of a sulfur catalyst. Iron gives iron pentacarbonyl
too, but this reaction is slow. If necessary, it may be separated by distillation. Dicobalt octacarbonyl
is also formed in this process, but it decomposes to tetracobalt dodecacarbonyl at the reaction temperature to give a non-volatile solid.
Nickel is re-obtained from the nickel carbonyl by one of two processes. It may be passed through a large chamber at high temperatures in which tens of thousands of nickel spheres, called pellets, are constantly stirred. It then decomposes depositing pure nickel onto the nickel spheres. Alternatively, the nickel carbonyl may be decomposed in a smaller chamber at 230 °C to create fine nickel powder. The resultant carbon monoxide is re-circulated and reused through the process. The highly pure nickel produced by this process is known as "carbonyl nickel."
or 1.47 USD/oz. The price subsequently fell dramatically from these peaks, and as of 19 January 2009 the metal was trading at 10,880 USD/tonne.
The US nickel coin
contains 0.04 oz (1.25 g) of nickel, which at the April 2007 price was worth 6.5 cents, along with 3.75 grams of copper worth about 3 cents, making the metal value over 9 cents. Since the face value of a nickel is 5 cents, this made it an attractive target for melting by people wanting to sell the metals at a profit. However, the United States Mint
, in anticipation of this practice, implemented new interim rules on December 14, 2006, subject to public comment for 30 days, which criminalize the melting and export of cents and nickels. Violators can be punished with a fine of up to $10,000 and/or imprisoned for a maximum of five years.
As of September 16, 2011, the melt value of a U.S. nickel is $0.0600409, which is 20% higher than the face value.
 The fraction of global nickel production presently used for various applications is as follows: 60% for making nickel steels; 14% in nickel-copper alloys and nickel silver
The fraction of global nickel production presently used for various applications is as follows: 60% for making nickel steels; 14% in nickel-copper alloys and nickel silver
; 9% to make malleable nickel, nickel clad, Inconel
, and other superalloys; 6% in plating; 3% for nickel cast irons; 3% in heat and electric resistance alloys, such as Nichrome
; 2% for nickel brasses and bronzes; 3% in all other applications combined.
Nickel is used in many specific and recognizable industrial and consumer products, including stainless steel
, alnico
magnets, coinage, rechargeable batteries, electric guitar strings, microphone capsules, and special alloys. It is also used for plating and as a green tint in glass. Nickel is preeminently an alloy metal, and its chief use is in the nickel steels and nickel cast irons, of which there are many varieties. It is also widely used in many other alloys, such as nickel brasses and bronzes, and alloys with copper, chromium, aluminium, lead, cobalt, silver, and gold (Inconel
, Incoloy, Monel
, Nimonic
).
Because of its resistance to corrosion, nickel has been occasionally used historically as a substitute for decorative silver. Nickel was also occasionally used in some countries after 1859 as a cheap coinage metal (see above) but beginning the later years of the 20th century has largely replaced by cheaper stainless steel
(i.e., iron) alloys, except notably in the United States.
Nickel is an excellent alloying agent for certain other precious metals, and so used in the so-called fire assay, as a collector of platinum group elements
(PGE). As such, nickel is capable of full collection of all 6 PGE elements from ores, in addition to partial collection of gold. High-throughput nickel mines may also engage in PGE recovery (primarily platinum
and palladium
); examples are Norilsk in Russia and the Sudbury Basin in Canada.
Nickel foam
or nickel mesh
is used in gas diffusion electrode
s for alkaline fuel cell
s.
Nickel and its alloys are frequently used as catalysts for hydrogenation
reactions. Raney nickel
, a finely-divided nickel-aluminium alloy, is one common form, however related catalysts are also often used, including related 'Raney-type' catalysts.
Nickel is a naturally magnetostrictive material, meaning that, in the presence of a magnetic field
, the material undergoes a small change in length. In the case of nickel, this change in length is negative (contraction of the material), which is known as negative magnetostriction
and is on the order of 50 ppm.
Nickel is used as a binder in the cemented tungsten carbide or hardmetal industry and used in proportions of six to 12% by weight. Nickel can make the tungsten carbide magnetic and adds corrosion-resistant properties to the cemented tungsten carbide parts, although the hardnesses are lower than those of parts made of the binder cobalt.
(an enzyme that assists in the hydrolysis of urea
) contains nickel. The NiFe-hydrogenase
s contain nickel in addition to iron-sulfur cluster
s. Such [NiFe]-hydrogenases characteristically oxidise H2. A nickel-tetrapyrrole coenzyme, Cofactor F430
, is present in the methyl coenzyme M
reductase, which powers methanogen
ic archaea
. One of the carbon monoxide dehydrogenase enzymes consists of an Fe-Ni-S cluster. Other nickel-containing enzymes include a rare bacterial class of superoxide dismutase
and glyoxalase I enzymes in bacteria and several parasitic eukaryotic trypanosomal parasites
(this enzyme in higher organisms, including yeast and mammals, uses divalent zinc
, Zn2+).
ic, and various other nickel compounds may be as well. Nickel carbonyl
, [Ni(CO)4], is an extremely toxic gas. The toxicity of metal carbonyls is a function of both the toxicity of the metal as well as the carbonyl's ability to give off highly toxic carbon monoxide
gas, and this one is no exception; nickel carbonyl is also explosive in air.
Sensitized
individuals may show an allergy
to nickel, affecting their skin, also known as dermatitis
. Sensitivity to nickel may also be present in patients with pompholyx
. Nickel is an important cause of contact allergy, partly due to its use in jewellery intended for pierced ears. Nickel allergies affecting pierced ears are often marked by itchy, red skin. Many earrings are now made nickel-free due to this problem. The amount of nickel allowed in products that come into contact with human skin is regulated by the European Union
. In 2002, researchers found amounts of nickel being emitted by 1 and 2 Euro coins far in excess of those standards. This is believed to be due to a galvanic
reaction.
It was voted Allergen of the Year
in 2008 by the American Contact Dermatitis Society.
Reports also showed that both the nickel-induced activation of hypoxia-inducible factor (HIF-1) and the up-regulation of hypoxia-inducible genes are due to depleted intracellular ascorbate levels. The addition of ascorbate to the culture medium increased the intracellular ascorbate level and reversed both the metal-induced stabilization of HIF-1- and HIF-1α-dependent gene expression.
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
with the chemical symbol
Chemical symbol
A chemical symbol is a 1- or 2-letter internationally agreed code for a chemical element, usually derived from the name of the element, often in Latin. Only the first letter is capitalised...
Ni and atomic number
Atomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
28. It is a silvery-white lustrous metal
Metal
A metal , is an element, compound, or alloy that is a good conductor of both electricity and heat. Metals are usually malleable and shiny, that is they reflect most of incident light...
with a slight golden tinge. Nickel belongs to the transition metals and is hard and ductile
Ductility
In materials science, ductility is a solid material's ability to deform under tensile stress; this is often characterized by the material's ability to be stretched into a wire. Malleability, a similar property, is a material's ability to deform under compressive stress; this is often characterized...
. Pure nickel shows a significant chemical activity, though larger pieces of the metal are slow to react with air at ambient conditions due to the formation of a protective oxide surface. However, nickel is reactive with oxygen to the extent that native
Native Metal
A native metal is any metal that is found in its metallic form, either pure or as an alloy, in nature. Metals that can be found as native deposits singly and/or in alloys include aluminium, antimony, arsenic, bismuth, cadmium, chromium, cobalt, indium, iron, nickel, selenium, tantalum, tellurium,...
nickel is rare on Earth's surface, and is mostly confined to the interiors of larger nickel iron meteorites, which were protected from oxidation in space. Such native nickel is always found on Earth alloyed with iron, in keeping with the element's origin as a major end-product of the nucleosynthesis
Nucleosynthesis
Nucleosynthesis is the process of creating new atomic nuclei from pre-existing nucleons . It is thought that the primordial nucleons themselves were formed from the quark–gluon plasma from the Big Bang as it cooled below two trillion degrees...
process, along with iron, in supernovas. An iron-nickel alloy is thought to compose the Earth's core
Inner core
The inner core of the Earth, its innermost hottest part as detected by seismological studies, is a primarily solid ball about in radius, or about 70% that of the Moon...
.
The use of nickel (as a natural meteoric nickel-iron alloy) has been traced as far back as 3500 BC. Nickel was first isolated and classified as a chemical element in 1751 by Axel Fredrik Cronstedt
Axel Fredrik Cronstedt
Baron Axel Fredrik Cronstedt was a Swedish mineralogist and chemist who discovered nickel in 1751 as a mining expert with the Bureau of Mines. Cronstedt described it as kupfernickel...
, who initially mistook its ore
Ore
An ore is a type of rock that contains minerals with important elements including metals. The ores are extracted through mining; these are then refined to extract the valuable element....
for a copper mineral. Its most important ore minerals are laterite
Laterite
Laterites are soil types rich in iron and aluminium, formed in hot and wet tropical areas. Nearly all laterites are rusty-red because of iron oxides. They develop by intensive and long-lasting weathering of the underlying parent rock...
s, including limonite
Limonite
Limonite is an ore consisting in a mixture of hydrated iron oxide-hydroxide of varying composition. The generic formula is frequently written as FeO·nH2O, although this is not entirely accurate as limonite often contains a varying amount of oxide compared to hydroxide.Together with hematite, it has...
, garnierite
Garnierite
Garnierite is a general name for a green nickel ore which is found in pockets and veins within weathered and serpentinized ultramafic rocks. It forms by lateritic weathering of ultramafic rocks and occurs in many nickel laterite deposits in the world. It is an important nickel ore, having a large...
, and pentlandite
Pentlandite
Pentlandite is an iron-nickel sulfide, 9S8. Pentlandite usually has a Ni:Fe ratio of close to 1:1. It also contains minor cobalt.Pentlandite forms isometric crystals, but is normally found in massive granular aggregates. It is brittle with a hardness of 3.5 - 4 and specific gravity of 4.6 - 5.0 and...
. Major production sites include Sudbury region
Sudbury Basin
The Sudbury Basin, also known as Sudbury Structure or the Sudbury Nickel Irruptive, is a major geologic structure in Ontario, Canada. It is the second-largest known impact crater or astrobleme on Earth, as well as one of the oldest....
in Canada (which is thought to be of meteoric origin), New Caledonia
New Caledonia
New Caledonia is a special collectivity of France located in the southwest Pacific Ocean, east of Australia and about from Metropolitan France. The archipelago, part of the Melanesia subregion, includes the main island of Grande Terre, the Loyalty Islands, the Belep archipelago, the Isle of...
and Norilsk
Norilsk
Norilsk is an industrial city in Krasnoyarsk Krai, Russia, located between the Yenisei River and the Taymyr Peninsula. Population: It was granted city status in 1953. It is the northernmost city in Siberia and the world's second largest city north of the Arctic Circle...
in Russia.
Because of nickel's slow rate of oxidation at room temperature, it is considered corrosion-resistant. Historically this has led to its use for plating metals such as iron and brass
Brass
Brass is an alloy of copper and zinc; the proportions of zinc and copper can be varied to create a range of brasses with varying properties.In comparison, bronze is principally an alloy of copper and tin...
, to its use for chemical apparatus, and its use in certain alloys that will retain a high silvery polish, such as German silver
Nickel silver
Nickel silver, also known as German silver, Argentann, new silver, nickel brass, albata,, or alpacca, is a copper alloy with nickel and often zinc. The usual formulation is 60% copper, 20% nickel and 20% zinc. Nickel silver is named for its silvery appearance, but it contains no elemental silver...
. About 6% of world nickel production is still used for corrosion-resistant pure-nickel plating. Nickel was once a common component of coins, but has largely been replaced by cheaper iron for this purpose, especially since the metal has proven to be a skin allergen
Allergen
An allergen is any substance that can cause an allergy. In technical terms, an allergen is a non-parasitic antigen capable of stimulating a type-I hypersensitivity reaction in atopic individuals....
for some people.
Nickel is one of the four elements that are ferromagnetic around room temperature. Alnico
Alnico
Alnico is an acronym referring to iron alloys which in addition to iron are composed primarily of aluminium , nickel and cobalt , hence al-ni-co, with the addition of copper, and sometimes titanium. Alnico alloys are ferromagnetic, with a high coercivity and are used to make permanent magnets...
permanent magnets based partly on nickel are of intermediate strength between iron-based permanent magnets and rare earth magnets
Rare-earth magnet
Rare-earth magnets are strong permanent magnets made from alloys of rare earth elements. Developed in the 1970s and 80s, rare-earth magnets are the strongest type of permanent magnets made and have significant performance advantages over ferrite or alnico magnets...
. The metal is chiefly valuable in the modern world for the alloy
Alloy
An alloy is a mixture or metallic solid solution composed of two or more elements. Complete solid solution alloys give single solid phase microstructure, while partial solutions give two or more phases that may or may not be homogeneous in distribution, depending on thermal history...
s it forms; about 60% of world production is used in nickel-steels (particularly stainless steel
Stainless steel
In metallurgy, stainless steel, also known as inox steel or inox from French "inoxydable", is defined as a steel alloy with a minimum of 10.5 or 11% chromium content by mass....
). Other common alloys, as well as some new superalloy
Superalloy
A superalloy, or high-performance alloy, is an alloy that exhibits excellent mechanical strength and creep resistance at high temperatures, good surface stability, and corrosion and oxidation resistance. Superalloys typically have a matrix with an austenitic face-centered cubic crystal structure. ...
s, make up most of the remainder of world nickel use, with chemical uses for nickel compounds consuming less than 3% of production. As a compound, nickel has a number of niche chemical manufacturing uses, such as a catalyst for hydrogenation
Raney nickel
Raney nickel is a solid catalyst composed of fine grains of a nickel-aluminium alloy, used in many industrial processes. It was developed in 1926 by American]] engineer Murray Raney as an alternative catalyst for the hydrogenation of vegetable oils in industrial processes...
. Enzymes of some microorganisms and plants contain nickel as an active center, which makes the metal an essential nutrient for them.
Atomic and physical properties
Nickel is a silvery-white metal with a slight golden tinge that takes a high polish. It is one of only four elements that are magnetic at or near room temperature, the others being iron, cobaltCobalt
Cobalt is a chemical element with symbol Co and atomic number 27. It is found naturally only in chemically combined form. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal....
and gadolinium
Gadolinium
Gadolinium is a chemical element with the symbol Gd and atomic number 64. It is a silvery-white, malleable and ductile rare-earth metal. It is found in nature only in combined form. Gadolinium was first detected spectroscopically in 1880 by de Marignac who separated its oxide and is credited with...
. Its Curie temperature is 355 °C, meaning that bulk nickel is non-magnetic above this temperature. The unit cell of nickel is a face centered cube
Cubic crystal system
In crystallography, the cubic crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals....
with the lattice parameter of 0.352 nm giving an atomic radius
Atomic radius
The atomic radius of a chemical element is a measure of the size of its atoms, usually the mean or typical distance from the nucleus to the boundary of the surrounding cloud of electrons...
of 0.124 nm. Nickel belongs to the transition metals and is hard and ductile
Ductility
In materials science, ductility is a solid material's ability to deform under tensile stress; this is often characterized by the material's ability to be stretched into a wire. Malleability, a similar property, is a material's ability to deform under compressive stress; this is often characterized...
.
The nickel atom has two electron configuration
Electron configuration
In atomic physics and quantum chemistry, electron configuration is the arrangement of electrons of an atom, a molecule, or other physical structure...
s, [Ar] 4s2 3d8 and [Ar] 4s1 3d9, which are very close in energy, where the symbol [Ar] refers to the argon
Argon
Argon is a chemical element represented by the symbol Ar. Argon has atomic number 18 and is the third element in group 18 of the periodic table . Argon is the third most common gas in the Earth's atmosphere, at 0.93%, making it more common than carbon dioxide...
-like core structure. There is some disagreement as to which should be considered the lowest energy configuration. Chemistry textbooks quote the electron configuration of nickel as [Ar] 4s2 3d8, or equivalently as [Ar] 3d8 4s2. This configuration agrees with the Madelung energy ordering rule, which predicts that 4s is filled before 3d. It is supported by the experimental fact that the lowest energy state of the nickel atom is a 4s2 3d8 energy level, specifically the 3d8(3F) 4s2 3F, J=4 level.
However each of these two configurations in fact gives rise to a set of states at different energies. The two sets of energies overlap, and the average energy of states having configuration [Ar] 4s1 3d9 is in fact lower than the average energy of states having configuration [Ar] 4s2 3d8. For this reason the research literature on atomic calculations quotes the ground state configuration of nickel as 4s1 3d9.
Isotopes
Naturally occurring nickel is composed of 5 stable isotopeIsotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
s; 58Ni, 60Ni, 61Ni, 62Ni and 64Ni with 58Ni being the most abundant (68.077% natural abundance
Natural abundance
In chemistry, natural abundance refers to the abundance of isotopes of a chemical element as naturally found on a planet. The relative atomic mass of these isotopes is the atomic weight listed for the element in the periodic table...
). 62Ni
Nickel-62
Nickel-62 is an isotope of nickel having 28 protons and 34 neutrons.It is a stable isotope, with the highest binding energy per nucleon of any known nuclide . It is often stated that 56Fe is the "most stable nucleus", but actually 56Fe has the lowest mass per nucleon of all nuclides...
is the "most stable" nuclide of all the existing elements, with binding energy greater than both 56Fe
Iron-56
Iron-56 is the most common isotope of iron. About 91.754% of all iron is iron-56.Of all isotopes, iron-56 has the lowest mass per nucleon. With 8.8 MeV binding energy per nucleon, iron-56 is one of the most tightly bound nuclei....
, often incorrectly cited as "most stable", and 58Fe. 18 radioisotopes have been characterised with the most stable being 59Ni with a half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of 76,000 years, 63Ni with a half-life of 100.1 years, and 56Ni with a half-life of 6.077 days. All of the remaining radioactive isotopes have half-lives that are less than 60 hours and the majority of these have half-lives that are less than 30 seconds. This element also has 1 meta state.
Nickel-56 is produced by the silicon burning process
Silicon burning process
In astrophysics, silicon burning is a very brief sequence of nuclear fusion reactions that occur in massive stars with a minimum of about 8–11 solar masses. Silicon burning is the final stage of fusion for massive stars that have run out of the fuels that power them for their long lives in the main...
and later set free in large quantities during type Ia supernova
Supernova
A supernova is a stellar explosion that is more energetic than a nova. It is pronounced with the plural supernovae or supernovas. Supernovae are extremely luminous and cause a burst of radiation that often briefly outshines an entire galaxy, before fading from view over several weeks or months...
e. Indeed, the shape of the light curve
Light curve
In astronomy, a light curve is a graph of light intensity of a celestial object or region, as a function of time. The light is usually in a particular frequency interval or band...
of these supernovae at intermediate to late-times corresponds to the decay via electron capture
Electron capture
Electron capture is a process in which a proton-rich nuclide absorbs an inner atomic electron and simultaneously emits a neutrino...
of nickel-56 to cobalt
Cobalt
Cobalt is a chemical element with symbol Co and atomic number 27. It is found naturally only in chemically combined form. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal....
-56 and ultimately to iron-56. Nickel-59 is a long-lived cosmogenic
Cosmogenic nuclide
See also Environmental radioactivity#NaturalCosmogenic nuclides are rare isotopes created when a high-energy cosmic ray interacts with the nucleus of an in situ solar system atom, causing cosmic ray spallation...
radionuclide
Radionuclide
A radionuclide is an atom with an unstable nucleus, which is a nucleus characterized by excess energy available to be imparted either to a newly created radiation particle within the nucleus or to an atomic electron. The radionuclide, in this process, undergoes radioactive decay, and emits gamma...
with a half-life of 76,000 years. 59Ni has found many applications in isotope geology. 59Ni has been used to date the terrestrial age of meteorite
Meteorite
A meteorite is a natural object originating in outer space that survives impact with the Earth's surface. Meteorites can be big or small. Most meteorites derive from small astronomical objects called meteoroids, but they are also sometimes produced by impacts of asteroids...
s and to determine abundances of extraterrestrial dust in ice and sediment
Sediment
Sediment is naturally occurring material that is broken down by processes of weathering and erosion, and is subsequently transported by the action of fluids such as wind, water, or ice, and/or by the force of gravity acting on the particle itself....
. Nickel-60 is the daughter product of the extinct radionuclide 60Fe, which decays with a half-life of 2.6 million years. Because 60Fe has such a long half-life, its persistence in materials in the solar system
Solar System
The Solar System consists of the Sun and the astronomical objects gravitationally bound in orbit around it, all of which formed from the collapse of a giant molecular cloud approximately 4.6 billion years ago. The vast majority of the system's mass is in the Sun...
at high enough concentrations may have generated observable variations in the isotopic composition of 60Ni. Therefore, the abundance of 60Ni present in extraterrestrial material may provide insight into the origin of the solar system and its early history. Nickel-62
Nickel-62
Nickel-62 is an isotope of nickel having 28 protons and 34 neutrons.It is a stable isotope, with the highest binding energy per nucleon of any known nuclide . It is often stated that 56Fe is the "most stable nucleus", but actually 56Fe has the lowest mass per nucleon of all nuclides...
has the highest binding energy
Binding energy
Binding energy is the mechanical energy required to disassemble a whole into separate parts. A bound system typically has a lower potential energy than its constituent parts; this is what keeps the system together—often this means that energy is released upon the creation of a bound state...
per nucleon of any isotope for any element (8.7946 Mev/nucleon). Isotopes heavier than 62Ni cannot be formed by nuclear fusion
Nuclear fusion
Nuclear fusion is the process by which two or more atomic nuclei join together, or "fuse", to form a single heavier nucleus. This is usually accompanied by the release or absorption of large quantities of energy...
without losing energy. Nickel-48, discovered in 1999, is the most proton-rich heavy element isotope known. With 28 proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
s and 20 neutron
Neutron
The neutron is a subatomic hadron particle which has the symbol or , no net electric charge and a mass slightly larger than that of a proton. With the exception of hydrogen, nuclei of atoms consist of protons and neutrons, which are therefore collectively referred to as nucleons. The number of...
s 48Ni is "double magic" (like 208Pb
Lead
Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
) and therefore unusually stable.
The isotopes of nickel range in atomic weight
Atomic weight
Atomic weight is a dimensionless physical quantity, the ratio of the average mass of atoms of an element to 1/12 of the mass of an atom of carbon-12...
from 48 u
Atomic mass unit
The unified atomic mass unit or dalton is a unit that is used for indicating mass on an atomic or molecular scale. It is defined as one twelfth of the rest mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state, and has a value of...
to 78 u . Nickel-78's half-life was recently measured to be 110 milliseconds and is believed to be an important isotope involved in supernova nucleosynthesis
Supernova nucleosynthesis
Supernova nucleosynthesis is the production of new chemical elements inside supernovae. It occurs primarily due to explosive nucleosynthesis during explosive oxygen burning and silicon burning...
of elements heavier than iron.
Occurrence
On Earth, nickel occurs most often in combination with sulfurSulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
and iron in pentlandite
Pentlandite
Pentlandite is an iron-nickel sulfide, 9S8. Pentlandite usually has a Ni:Fe ratio of close to 1:1. It also contains minor cobalt.Pentlandite forms isometric crystals, but is normally found in massive granular aggregates. It is brittle with a hardness of 3.5 - 4 and specific gravity of 4.6 - 5.0 and...
, with sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
in millerite
Millerite
Millerite is a nickel sulfide mineral, NiS. It is brassy in colour and has an acicular habit, often forming radiating masses and furry aggregates...
, with arsenic
Arsenic
Arsenic is a chemical element with the symbol As, atomic number 33 and relative atomic mass 74.92. Arsenic occurs in many minerals, usually in conjunction with sulfur and metals, and also as a pure elemental crystal. It was first documented by Albertus Magnus in 1250.Arsenic is a metalloid...
in the mineral nickeline, and with arsenic and sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
in nickel galena
Galena
Galena is the natural mineral form of lead sulfide. It is the most important lead ore mineral.Galena is one of the most abundant and widely distributed sulfide minerals. It crystallizes in the cubic crystal system often showing octahedral forms...
. Nickel is commonly found in iron meteorite
Iron meteorite
Iron meteorites are meteorites that consist overwhelmingly of nickel-iron alloys. The metal taken from these meteorites is known as meteoric iron and was one of the earliest sources of usable iron available to humans.-Occurrence:...
s as the alloys kamacite
Kamacite
Kamacite is a mineral. It is an alloy of iron and nickel, usually in the proportions of 90:10 to 95:5 although impurities such as cobalt or carbon may be present. On the surface of Earth, it occurs naturally only in meteorites. It has a metallic luster, is gray and has no clear cleavage although...
and taenite
Taenite
Taenite is a mineral found naturally on Earth mostly in iron meteorites. It is an alloy of iron and nickel, with nickel proportions of 20% up to 65%.The name is derived from the Greek for "band". Taenite is a major constituent of iron meteorites...
.
The bulk of the nickel mined comes from two types of ore
Ore
An ore is a type of rock that contains minerals with important elements including metals. The ores are extracted through mining; these are then refined to extract the valuable element....
deposits. The first are laterite
Laterite
Laterites are soil types rich in iron and aluminium, formed in hot and wet tropical areas. Nearly all laterites are rusty-red because of iron oxides. They develop by intensive and long-lasting weathering of the underlying parent rock...
s where the principal ore minerals are nickeliferous limonite
Limonite
Limonite is an ore consisting in a mixture of hydrated iron oxide-hydroxide of varying composition. The generic formula is frequently written as FeO·nH2O, although this is not entirely accurate as limonite often contains a varying amount of oxide compared to hydroxide.Together with hematite, it has...
: (Fe, Ni)O(OH) and garnierite
Garnierite
Garnierite is a general name for a green nickel ore which is found in pockets and veins within weathered and serpentinized ultramafic rocks. It forms by lateritic weathering of ultramafic rocks and occurs in many nickel laterite deposits in the world. It is an important nickel ore, having a large...
(a hydrous nickel silicate): (Ni, Mg)3Si2O5(OH)4. The second are magmatic sulfide deposits where the principal ore mineral is pentlandite
Pentlandite
Pentlandite is an iron-nickel sulfide, 9S8. Pentlandite usually has a Ni:Fe ratio of close to 1:1. It also contains minor cobalt.Pentlandite forms isometric crystals, but is normally found in massive granular aggregates. It is brittle with a hardness of 3.5 - 4 and specific gravity of 4.6 - 5.0 and...
: (Ni, Fe)9S8.
In terms of supply, the Sudbury region of Ontario
Ontario
Ontario is a province of Canada, located in east-central Canada. It is Canada's most populous province and second largest in total area. It is home to the nation's most populous city, Toronto, and the nation's capital, Ottawa....
, Canada, produces about 30% of the world's supply of nickel. The Sudbury Basin deposit is theorized to have been created by a meteorite
Meteorite
A meteorite is a natural object originating in outer space that survives impact with the Earth's surface. Meteorites can be big or small. Most meteorites derive from small astronomical objects called meteoroids, but they are also sometimes produced by impacts of asteroids...
impact event
Impact event
An impact event is the collision of a large meteorite, asteroid, comet, or other celestial object with the Earth or another planet. Throughout recorded history, hundreds of minor impact events have been reported, with some occurrences causing deaths, injuries, property damage or other significant...
early in the geologic history of Earth
History of Earth
The history of the Earth describes the most important events and fundamental stages in the development of the planet Earth from its formation 4.578 billion years ago to the present day. Nearly all branches of natural science have contributed to the understanding of the main events of the Earth's...
. Russia contains about 40% of the world's known resources at the Norilsk deposit in Siberia
Siberia
Siberia is an extensive region constituting almost all of Northern Asia. Comprising the central and eastern portion of the Russian Federation, it was part of the Soviet Union from its beginning, as its predecessor states, the Tsardom of Russia and the Russian Empire, conquered it during the 16th...
. The Russian mining company MMC Norilsk Nickel
MMC Norilsk Nickel
MMC Norilsk Nickel is a nickel and palladium mining and smelting company. Its largest operations are located in the Norilsk–Talnakh area, in northern Russia. MMC stands for "Mining and Metallurgical Company"....
obtains the nickel and the associated palladium
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
for world distribution. Other major deposits of nickel are found in New Caledonia
New Caledonia
New Caledonia is a special collectivity of France located in the southwest Pacific Ocean, east of Australia and about from Metropolitan France. The archipelago, part of the Melanesia subregion, includes the main island of Grande Terre, the Loyalty Islands, the Belep archipelago, the Isle of...
, France, Australia, Cuba, and Indonesia. Deposits found in tropical areas typically consist of laterites, which are produced by the intense weathering of ultramafic igneous rocks and the resulting secondary concentration of nickel bearing oxide and silicate minerals
Silicate minerals
The silicate minerals make up the largest and most important class of rock-forming minerals, constituting approximately 90 percent of the crust of the Earth. They are classified based on the structure of their silicate group...
.
Based on geophysical
Geophysics
Geophysics is the physics of the Earth and its environment in space; also the study of the Earth using quantitative physical methods. The term geophysics sometimes refers only to the geological applications: Earth's shape; its gravitational and magnetic fields; its internal structure and...
evidence, most of the nickel on Earth is postulated to be concentrated in the Earth's core. Kamacite
Kamacite
Kamacite is a mineral. It is an alloy of iron and nickel, usually in the proportions of 90:10 to 95:5 although impurities such as cobalt or carbon may be present. On the surface of Earth, it occurs naturally only in meteorites. It has a metallic luster, is gray and has no clear cleavage although...
and taenite
Taenite
Taenite is a mineral found naturally on Earth mostly in iron meteorites. It is an alloy of iron and nickel, with nickel proportions of 20% up to 65%.The name is derived from the Greek for "band". Taenite is a major constituent of iron meteorites...
are naturally occurring alloy
Alloy
An alloy is a mixture or metallic solid solution composed of two or more elements. Complete solid solution alloys give single solid phase microstructure, while partial solutions give two or more phases that may or may not be homogeneous in distribution, depending on thermal history...
s of iron and nickel. For kamacite the alloy is usually in the proportion of 90:10 to 95:5 although impurities such as cobalt
Cobalt
Cobalt is a chemical element with symbol Co and atomic number 27. It is found naturally only in chemically combined form. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal....
or carbon
Carbon
Carbon is the chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds...
may be present, while for taenite the nickel content is between 20% and 65%. Kamacite and taenite occur in nickel iron meteorites.
Compounds

Oxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
of nickel is +2, but compounds of Ni0, Ni+, and Ni3+ are well known, and Ni4+ has been demonstrated.
Nickel(0)
Tetracarbonylnickel (Ni(CO)4), discovered by Ludwig MondLudwig Mond
Dr Ludwig Mond , was a German-born chemist and industrialist who took British nationality.-Education and career:...
, is a volatile liquid at room temperature. On heating, the complex decomposes back to nickel and carbon monoxide:
- Ni(CO)4
 Ni + 4 CO
Ni + 4 CO
This behavior is exploited in the Mond process
Mond process
The Mond process, sometimes known as the carbonyl process is a technique created by Ludwig Mond in 1890 to extract and purify nickel. The process was used commercially before the end of the 19th century...
for purifying nickel, as described above. The related nickel(0) complex bis(cyclooctadiene)nickel(0)
Bis(cyclooctadiene)nickel(0)
Bisnickel is the organometallic compound with the formula Ni2. This air-sensitive yellow solid is a common source of Ni in chemical synthesis....
is a useful catalyst in organonickel chemistry due to the easily displaced cod ligands.
Nickel(II)
-sulfate-hexahydrate-sample.jpg)
Nickel(II) sulfate
Nickel sulfate, or just nickel sulfate, usually refers to the inorganic compound with the formula NiSO46. This highly soluble blue-coloured salt is a common source of the Ni2+ ion for electroplating. Nickel sulfate is paramagnetic....
is produced in large quantities by dissolving nickel metal or oxides in sulfuric acid. It exists as both a hexa- and heptahydrates. This compound is useful for electroplating
Electroplating
Electroplating is a plating process in which metal ions in a solution are moved by an electric field to coat an electrode. The process uses electrical current to reduce cations of a desired material from a solution and coat a conductive object with a thin layer of the material, such as a metal...
nickel.
The four halogens form nickel compounds, all of which adopt octahedral geometries. Nickel(II) chloride
Nickel(II) chloride
Nickel chloride , is the chemical compound NiCl2. The anhydrous salt is yellow, but the more familiar hydrate NiCl2·6H2O is green. It is very rarely found in nature as mineral nickelbischofite. A dihydrate is also known. In general nickel chloride, in various forms, is the most important source of...
is most common, and its behavior is illustrative of the other halides. Nickel(II) chloride is produced by dissolving nickel residues in hydrochloric acid. The dichloride is usually encountered as the green hexahydrate, but it can be dehydrated to give the yellow anhydrous NiCl2. Some tetracoordinate nickel(II) complexes form both tetrahedral and square planar geometries. The tetrahedral complexes are paramagnetic and the square planar complexes are diamagnetic. This equilibrium as well as the formation of octahedral complexes contrasts with the behavior of the divalent complexes of the heavier group 10 metals, palladium(II) and platinum(II), which tend to adopt only square-planar complexes.
Nickelocene
Nickelocene
Nickelocene is the organonickel compound with the formula Ni2. Also known as bisnickel or NiCp2, this bright green paramagnetic solid is of enduring academic interest, although it yet has no practical applications....
is known; it has an electron count of 20, making it relatively unstable.
Nickel(I), (III), and (IV)
For simple compounds, nickel(III) and nickel(IV) only occurs with fluoride and oxides. Nickel(III) oxideNickel(III) oxide
Nickel oxide has been referred to in the literature but is not a well characterised compound. The substance black nickel oxide is sometimes described as being Ni2O3 however the composition quoted by suppliers has a nickel content of around 77% by weight whereas Ni2O3 would have 70.98% Ni by...
is used as the cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
in many rechargeable batteries, including nickel-cadmium, nickel-iron
Nickel-iron battery
The nickel–iron battery is a storage battery having a nickel oxide-hydroxide cathode and an iron anode, with an electrolyte of potassium hydroxide. The active materials are held in nickel-plated steel tubes or perforated pockets...
, nickel hydrogen
Nickel hydrogen battery
A nickel–hydrogen battery is a rechargeable electrochemical power source based on nickel and hydrogen. It differs from a nickel–metal hydride battery by the use of hydrogen in a pressurized cell at up to 1200 psi pressure.The cathode is made up of a dry sintered porous nickel plaque, which...
, and nickel-metal hydride, and used by certain manufacturers in Li-ion batteries.
History
Because the ores of nickel are easily mistaken for ores of silver, understanding of this metal and its use dates to relatively recent times. However, the unintentional use of nickel is ancient, and can be traced back as far as 3500 BC. BronzeBronze
Bronze is a metal alloy consisting primarily of copper, usually with tin as the main additive. It is hard and brittle, and it was particularly significant in antiquity, so much so that the Bronze Age was named after the metal...
s from what is now Syria had contained up to 2% nickel. Further, there are Chinese manuscripts suggesting that "white copper" (cupronickel
Cupronickel
Cupronickel or copper-nickel or "cupernickel" is an alloy of copper that contains nickel and strengthening elements, such as iron and manganese. Cupronickel is highly resistant to corrosion in seawater, because its electrode potential is adjusted to be neutral with regard to seawater...
, known as baitung) was used there between 1700 and 1400 BC. This Paktong white copper was exported to Britain as early as the 17th century, but the nickel content of this alloy was not discovered until 1822.
In medieval Germany, a red mineral was found in the Erzgebirge (Ore Mountains) that resembled copper ore. However, when miners were unable to extract any copper from it, they blamed a mischievous sprite of German mythology, Nickel (similar to Old Nick), for besetting the copper. They called this ore Kupfernickel from the German Kupfer for copper. This ore is now known to be nickeline or niccolite
Niccolite
Nickeline or niccolite is a mineral consisting of nickel arsenide, NiAs, containing 43.9% nickel and 56.1% arsenic.Small quantities of sulfur, iron and cobalt are usually present, and sometimes the arsenic is largely replaced by antimony...
, a nickel arsenide
Arsenide
Arsenide is an arsenic anion with the charge −3. The trianion is formed by the reduction of arsenic by three electrons. For example heating arsenic powder with excess sodium gives sodium arsenide . The anions have no existence in solution since they are extremely basic...
. In 1751, Baron Axel Fredrik Cronstedt
Axel Fredrik Cronstedt
Baron Axel Fredrik Cronstedt was a Swedish mineralogist and chemist who discovered nickel in 1751 as a mining expert with the Bureau of Mines. Cronstedt described it as kupfernickel...
was attempting to extract copper from kupfernickel and obtained instead a white metal that he named after the spirit which had given its name to the mineral, nickel. In modern German, Kupfernickel or Kupfer-Nickel designates the alloy cupronickel
Cupronickel
Cupronickel or copper-nickel or "cupernickel" is an alloy of copper that contains nickel and strengthening elements, such as iron and manganese. Cupronickel is highly resistant to corrosion in seawater, because its electrode potential is adjusted to be neutral with regard to seawater...
.
After its discovery, the only source for nickel was the rare Kupfernickel, but, from 1824 on, the nickel was obtained as byproduct of cobalt blue
Cobalt blue
Cobalt blue is a cool, slightly desaturated blue color, historically made using cobalt salts of alumina. It is used in certain ceramics and painting; the different cobalt pigment smalt, based on silica, is more often used directly in tinted transparent glasses...
production. The first large-scale producer of nickel was Norway, which exploited nickel-rich pyrrhotite
Pyrrhotite
Pyrrhotite is an unusual iron sulfide mineral with a variable iron content: FeS . The FeS endmember is known as troilite. Pyrrhotite is also called magnetic pyrite because the color is similar to pyrite and it is weakly magnetic...
from 1848 on. The introduction of nickel in steel production in 1889 increased the demand for nickel, and the nickel deposits of New Caledonia
New Caledonia
New Caledonia is a special collectivity of France located in the southwest Pacific Ocean, east of Australia and about from Metropolitan France. The archipelago, part of the Melanesia subregion, includes the main island of Grande Terre, the Loyalty Islands, the Belep archipelago, the Isle of...
, which were discovered in 1865, provided most of the world's supply between 1875 and 1915. The discovery of the large deposits in the Sudbury Basin, Canada in 1883, in Norilsk-Talnakh
Norilsk
Norilsk is an industrial city in Krasnoyarsk Krai, Russia, located between the Yenisei River and the Taymyr Peninsula. Population: It was granted city status in 1953. It is the northernmost city in Siberia and the world's second largest city north of the Arctic Circle...
, Russia in 1920, and in the Merensky Reef
Merensky Reef
The Merensky Reef, is a layer of igneous rock in the Bushveld Igneous Complex in the Transvaal which together with an underlying layer, the Upper Group 2 Reef , contains most of the world's known reserves of platinum group metals or platinum group elements - platinum, palladium, rhodium,...
, South Africa in 1924 made large-scale production of nickel possible.
Nickel has been a component of coins since the mid-19th century. In the United States, the term "nickel" or "nick" was originally applied to the copper-nickel Flying Eagle cent
Flying Eagle cent
The Flying Eagle cent is a United States coin that was minted from 1856 to 1858. The coin was designed by James B. Longacre. The Flying Eagle was the first small-sized cent coin minted in the US, replacing the earlier large cent. The obverse of the coin depicts an eagle in flight, a unique subject...
, which replaced copper with 12% nickel 1857–58, then the Indian Head cent
Indian Head cent
The Indian Head one-cent coin, also known as an Indian Penny , was produced by the United States Mint from 1859 to 1909 at the Philadelphia Mint and in 1908 and 1909 at the San Francisco Mint...
of the same alloy from 1859–1864. Still later in 1865, the term designated the three-cent nickel
Three-cent piece (United States coin)
The United States three cent piece was a unit of currency equaling 3/100th of a United States dollar. The mint produced two different three-cent coins: the three-cent silver and the three-cent nickel. Its purchasing power in 1851 would be equivalent to $ today.-History:The three cent coin has an...
, with nickel increased to 25%. In 1866, the five-cent shield nickel
Nickel (United States coin)
The nickel is a five-cent coin, representing a unit of currency equaling five hundredths of one United States dollar. A later-produced Canadian nickel five-cent coin was also called by the same name....
(25% nickel, 75% copper) appropriated the designation. Along with the alloy proportion, this term has been used to the present in the United States. Coins of nearly pure nickel were first used in 1881 in Switzerland, and more notably 99.9% nickel five-cent coins
Nickel (Canadian coin)
The Canadian five-cent coin, commonly called a nickel, is a coin worth five cents or one-twentieth of a Canadian dollar. It was patterned on the corresponding coin in the neighbouring United States...
were struck in Canada (the world's largest nickel producer at the time) during non-war years from 1922–1981, and their metal content made these coins magnetic. During the wartime period 1942–45, more or all nickel was removed from Canadian and U.S. coins, due to nickel's war-critical use in armor. Canada switched alloys again to plated steel during the Korean war
Korean War
The Korean War was a conventional war between South Korea, supported by the United Nations, and North Korea, supported by the People's Republic of China , with military material aid from the Soviet Union...
, but was forced to stop making pure nickel "nickels" in 1981, reserving the pure 99.9% nickel alloy after 1968 only to its higher-value coins. Finally, in the 21st century, with rising nickel prices, most countries that formerly used nickel in their coins have abandoned the metal for cost reasons, and the U.S. five-cent coin remains one of the few in which the metal is still used, save for exterior plating.
Production
2.png)
British Geological Survey
The British Geological Survey is a partly publicly funded body which aims to advance geoscientific knowledge of the United Kingdom landmass and its continental shelf by means of systematic surveying, monitoring and research. The BGS headquarters are in Keyworth, Nottinghamshire, but other centres...
. A nickel deposit in western Turkey had been exploited, with this location being especially convenient for European smelters, steelmakers, and factories. The one locality in the United States where nickel was commercially mined is Riddle, Oregon
Riddle, Oregon
Riddle is a city in Douglas County, Oregon, United States. The population was 1,014 at the 2000 census.-Geography:According to the United States Census Bureau, the city has a total area of , all of it land.Riddle was founded by George Abner Riddle....
, where several square miles of nickel-bearing garnierite surface deposits are located. The mine closed in 1987. The Eagle mine project
Eagle mine project
The Eagle mine project is a proposed nickel and copper mine by Kennecott Minerals Corporation, a subsidiary of Rio Tinto. The company is currently considering opening the mine on the Yellow Dog Plains in Michigan's Upper Peninsula . Nickel and copper are the principal metals to be mined, but...
is a proposed new nickel mine in Michigan
Michigan
Michigan is a U.S. state located in the Great Lakes Region of the United States of America. The name Michigan is the French form of the Ojibwa word mishigamaa, meaning "large water" or "large lake"....
's upper peninsula
Upper Peninsula of Michigan
The Upper Peninsula of Michigan is the northern of the two major land masses that make up the U.S. state of Michigan. It is commonly referred to as the Upper Peninsula, the U.P., or Upper Michigan. It is also known as the land "above the Bridge" linking the two peninsulas. The peninsula is bounded...
.
Extraction and purification
Nickel is recovered through extractive metallurgyExtractive metallurgy
Extractive metallurgy is the study of the processes used in the separation and concentration of raw materials. The field is an applied science, covering all aspects of the physical and chemical processes used to produce mineral-containing and metallic materials, sometimes for direct use as a...
. Nickel is extracted from its ores by conventional roasting and reduction processes that yield a metal of greater than 75% purity. In many stainless steel
Stainless steel
In metallurgy, stainless steel, also known as inox steel or inox from French "inoxydable", is defined as a steel alloy with a minimum of 10.5 or 11% chromium content by mass....
applications, 75% pure nickel can be used without further purification, depending on the composition of the impurities.
Most sulfide ores have traditionally been processed using pyrometallurgical techniques to produce a matte
Matte (metallurgy)
Matte is a term used in the field of pyrometallurgy given to the molten metal sulfide phases typically formed during smelting of copper, nickel, and other base metals. Typically, a matte is the phase in which the principal metal being extracted is recovered prior to a final reduction process to...
for further refining. Recent advances in hydrometallurgy
Hydrometallurgy
Hydrometallurgy is part of the field of extractive metallurgy involving the use of aqueous chemistry for the recovery of metals from ores, concentrates, and recycled or residual materials...
have resulted in significant nickel purification using these processes. Most sulfide deposits have traditionally been processed by concentration through a froth flotation
Froth flotation
Froth flotation is a process for selectively separating hydrophobic materials from hydrophilic. This is used in several processing industries...
process followed by pyrometallurgical extraction. In hydrometallurgical processes, nickel sulfide ores undergo flotation (differential flotation if Ni/Fe ratio is too low) and then smelted. After producing the nickel matte, further processing is done via the Sherritt-Gordon process. First, copper is removed by adding hydrogen sulfide
Hydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
, leaving a concentrate of only cobalt and nickel. Then, solvent extraction is used to separate the cobalt and nickel, with the final nickel concentration greater than 99%.
Electrorefining
A second common form of further refining involves the leaching of the metal matte into a nickel salt solution, followed by the electro-winning of the nickel from solution by plating it onto a cathode as electrolytic nickel.Mond process
Purification of nickel oxides to obtain the purest metal is performed via the Mond processMond process
The Mond process, sometimes known as the carbonyl process is a technique created by Ludwig Mond in 1890 to extract and purify nickel. The process was used commercially before the end of the 19th century...
, which increases the nickel concentrate to greater than 99.99% purity. This process was patented by L. Mond and has been in industrial use since before the beginning of the 20th century. In the process, nickel is reacted with carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
at around 40–80 °C to form nickel carbonyl
Nickel carbonyl
Nickel carbonyl is the organonickel compound with the formula Ni4. This pale-yellow liquid is the principal carbonyl of nickel. It is an intermediate in the Mond process for the purification of nickel and a reagent in organometallic chemistry...
in the presence of a sulfur catalyst. Iron gives iron pentacarbonyl
Iron pentacarbonyl
Iron pentacarbonyl, also known as iron carbonyl, is the compound with formula 5. Under standard conditions Fe5 is a free-flowing, straw-colored liquid with a pungent odour. This compound is a common precursor to diverse iron compounds, including many that are useful in organic synthesis. Fe5 is...
too, but this reaction is slow. If necessary, it may be separated by distillation. Dicobalt octacarbonyl
Dicobalt octacarbonyl
Dicobalt octacarbonyl is the inorganic compound Co28. This metal carbonyl is a reagent and catalyst in organometallic chemistry and organic synthesis. It is used as a catalyst for hydroformylation, the conversion of alkenes to aldehydes....
is also formed in this process, but it decomposes to tetracobalt dodecacarbonyl at the reaction temperature to give a non-volatile solid.
Nickel is re-obtained from the nickel carbonyl by one of two processes. It may be passed through a large chamber at high temperatures in which tens of thousands of nickel spheres, called pellets, are constantly stirred. It then decomposes depositing pure nickel onto the nickel spheres. Alternatively, the nickel carbonyl may be decomposed in a smaller chamber at 230 °C to create fine nickel powder. The resultant carbon monoxide is re-circulated and reused through the process. The highly pure nickel produced by this process is known as "carbonyl nickel."
Metal value
The market price of nickel surged throughout 2006 and the early months of 2007; as of April 5, 2007, the metal was trading at 52,300 USD/tonneTonne
The tonne, known as the metric ton in the US , often put pleonastically as "metric tonne" to avoid confusion with ton, is a metric system unit of mass equal to 1000 kilograms. The tonne is not an International System of Units unit, but is accepted for use with the SI...
or 1.47 USD/oz. The price subsequently fell dramatically from these peaks, and as of 19 January 2009 the metal was trading at 10,880 USD/tonne.
The US nickel coin
Nickel (United States coin)
The nickel is a five-cent coin, representing a unit of currency equaling five hundredths of one United States dollar. A later-produced Canadian nickel five-cent coin was also called by the same name....
contains 0.04 oz (1.25 g) of nickel, which at the April 2007 price was worth 6.5 cents, along with 3.75 grams of copper worth about 3 cents, making the metal value over 9 cents. Since the face value of a nickel is 5 cents, this made it an attractive target for melting by people wanting to sell the metals at a profit. However, the United States Mint
United States Mint
The United States Mint primarily produces circulating coinage for the United States to conduct its trade and commerce. The Mint was created by Congress with the Coinage Act of 1792, and placed within the Department of State...
, in anticipation of this practice, implemented new interim rules on December 14, 2006, subject to public comment for 30 days, which criminalize the melting and export of cents and nickels. Violators can be punished with a fine of up to $10,000 and/or imprisoned for a maximum of five years.
As of September 16, 2011, the melt value of a U.S. nickel is $0.0600409, which is 20% higher than the face value.
Applications

Nickel silver
Nickel silver, also known as German silver, Argentann, new silver, nickel brass, albata,, or alpacca, is a copper alloy with nickel and often zinc. The usual formulation is 60% copper, 20% nickel and 20% zinc. Nickel silver is named for its silvery appearance, but it contains no elemental silver...
; 9% to make malleable nickel, nickel clad, Inconel
Inconel
Inconel is a registered trademark of Special Metals Corporation that refers to a family of austenitic nickel-chromium-based superalloys. Inconel alloys are typically used in high temperature applications. It is often referred to in English as "Inco"...
, and other superalloys; 6% in plating; 3% for nickel cast irons; 3% in heat and electric resistance alloys, such as Nichrome
Nichrome
Nichrome is a non-magnetic alloy of nickel, chromium, and often iron, usually used as a resistance wire. Patented in 1905, it is the oldest documented form of resistance heating alloy. A common alloy is 80% nickel and 20% chromium, by mass, but there are many others to accommodate various...
; 2% for nickel brasses and bronzes; 3% in all other applications combined.
Nickel is used in many specific and recognizable industrial and consumer products, including stainless steel
Stainless steel
In metallurgy, stainless steel, also known as inox steel or inox from French "inoxydable", is defined as a steel alloy with a minimum of 10.5 or 11% chromium content by mass....
, alnico
Alnico
Alnico is an acronym referring to iron alloys which in addition to iron are composed primarily of aluminium , nickel and cobalt , hence al-ni-co, with the addition of copper, and sometimes titanium. Alnico alloys are ferromagnetic, with a high coercivity and are used to make permanent magnets...
magnets, coinage, rechargeable batteries, electric guitar strings, microphone capsules, and special alloys. It is also used for plating and as a green tint in glass. Nickel is preeminently an alloy metal, and its chief use is in the nickel steels and nickel cast irons, of which there are many varieties. It is also widely used in many other alloys, such as nickel brasses and bronzes, and alloys with copper, chromium, aluminium, lead, cobalt, silver, and gold (Inconel
Inconel
Inconel is a registered trademark of Special Metals Corporation that refers to a family of austenitic nickel-chromium-based superalloys. Inconel alloys are typically used in high temperature applications. It is often referred to in English as "Inco"...
, Incoloy, Monel
Monel
Monel is a trademark of Special Metals Corporation for a series of nickel alloys, primarily composed of nickel and copper, with some iron and other trace elements. Monel was created by David H. Browne, chief metallurgist for International Nickel Co...
, Nimonic
Nimonic
Nimonic is a registered trademark of Special Metals Corporation that refers to a family of nickel-based superalloys. Nimonic alloys typically consist of more than 50% nickel and 20% chromium with additives such as titanium and aluminium. The main use is in gas turbine components and extremely high...
).
Because of its resistance to corrosion, nickel has been occasionally used historically as a substitute for decorative silver. Nickel was also occasionally used in some countries after 1859 as a cheap coinage metal (see above) but beginning the later years of the 20th century has largely replaced by cheaper stainless steel
Stainless steel
In metallurgy, stainless steel, also known as inox steel or inox from French "inoxydable", is defined as a steel alloy with a minimum of 10.5 or 11% chromium content by mass....
(i.e., iron) alloys, except notably in the United States.
Nickel is an excellent alloying agent for certain other precious metals, and so used in the so-called fire assay, as a collector of platinum group elements
Platinum group
The platinum group metals is a term used sometimes to collectively refer to six metallic elements clustered together in the periodic table.These elements are all transition metals, lying in the d-block .The six...
(PGE). As such, nickel is capable of full collection of all 6 PGE elements from ores, in addition to partial collection of gold. High-throughput nickel mines may also engage in PGE recovery (primarily platinum
Platinum
Platinum is a chemical element with the chemical symbol Pt and an atomic number of 78. Its name is derived from the Spanish term platina del Pinto, which is literally translated into "little silver of the Pinto River." It is a dense, malleable, ductile, precious, gray-white transition metal...
and palladium
Palladium
Palladium is a chemical element with the chemical symbol Pd and an atomic number of 46. It is a rare and lustrous silvery-white metal discovered in 1803 by William Hyde Wollaston. He named it after the asteroid Pallas, which was itself named after the epithet of the Greek goddess Athena, acquired...
); examples are Norilsk in Russia and the Sudbury Basin in Canada.
Nickel foam
Metal foam
A metal foam is a cellular structure consisting of a solid metal, frequently aluminium, containing a large volume fraction of gas-filled pores. The pores can be sealed , or they can form an interconnected network . The defining characteristic of metal foams is a very high porosity: typically...
or nickel mesh
Mesh
Mesh consists of semi-permeable barrier made of connected strands of metal, fiber, or other flexible/ductile material. Mesh is similar to web or net in that it has many attached or woven strands.-Types of mesh:...
is used in gas diffusion electrode
Gas diffusion electrode
Gas diffusion electrodes are electrodes with a conjunction of a solid, liquid and gaseous interface, and an electrical conducting catalyst supporting an electrochemical reaction between the liquid and the gaseous phase...
s for alkaline fuel cell
Alkaline fuel cell
The alkaline fuel cell , also known as the Bacon fuel cell after its British inventor, is one of the most developed fuel cell technologies. NASA has used alkaline fuel cells since the mid-1960s, in Apollo-series missions and on the Space Shuttle. AFCs consume hydrogen and pure oxygen producing...
s.
Nickel and its alloys are frequently used as catalysts for hydrogenation
Hydrogenation
Hydrogenation, to treat with hydrogen, also a form of chemical reduction, is a chemical reaction between molecular hydrogen and another compound or element, usually in the presence of a catalyst. The process is commonly employed to reduce or saturate organic compounds. Hydrogenation typically...
reactions. Raney nickel
Raney nickel
Raney nickel is a solid catalyst composed of fine grains of a nickel-aluminium alloy, used in many industrial processes. It was developed in 1926 by American]] engineer Murray Raney as an alternative catalyst for the hydrogenation of vegetable oils in industrial processes...
, a finely-divided nickel-aluminium alloy, is one common form, however related catalysts are also often used, including related 'Raney-type' catalysts.
Nickel is a naturally magnetostrictive material, meaning that, in the presence of a magnetic field
Magnetic field
A magnetic field is a mathematical description of the magnetic influence of electric currents and magnetic materials. The magnetic field at any given point is specified by both a direction and a magnitude ; as such it is a vector field.Technically, a magnetic field is a pseudo vector;...
, the material undergoes a small change in length. In the case of nickel, this change in length is negative (contraction of the material), which is known as negative magnetostriction
Magnetostriction
Magnetostriction is a property of ferromagnetic materials that causes them to change their shape or dimensions during the process of magnetization. The variation of material's magnetization due to the applied magnetic field changes the magnetostrictive strain until reaching its saturation value, λ...
and is on the order of 50 ppm.
Nickel is used as a binder in the cemented tungsten carbide or hardmetal industry and used in proportions of six to 12% by weight. Nickel can make the tungsten carbide magnetic and adds corrosion-resistant properties to the cemented tungsten carbide parts, although the hardnesses are lower than those of parts made of the binder cobalt.
Biological role
Although not recognized until the 1970s, nickel plays important roles in the biology of microorganisms and plants. In fact, ureaseUrease
Urease is an enzyme that catalyzes the hydrolysis of urea into carbon dioxide and ammonia. The reaction occurs as follows:In 1926, James Sumner showed that urease is a protein. Urease is found in bacteria, yeast, and several higher plants. The structure of urease was first solved by P.A...
(an enzyme that assists in the hydrolysis of urea
Urea
Urea or carbamide is an organic compound with the chemical formula CO2. The molecule has two —NH2 groups joined by a carbonyl functional group....
) contains nickel. The NiFe-hydrogenase
Hydrogenase
A hydrogenase is an enzyme that catalyses the reversible oxidation of molecular hydrogen . Hydrogenases play a vital role in anaerobic metabolism....
s contain nickel in addition to iron-sulfur cluster
Iron-sulfur cluster
For biological Fe-S clusters, see iron-sulfur proteins.Iron-sulfur clusters are ensembles of iron and sulfide centres. Fe-S clusters are most often discussed in the context of the biological role for iron-sulfur proteins. Many Fe-S clusters are known in the area of organometallic chemistry and as...
s. Such [NiFe]-hydrogenases characteristically oxidise H2. A nickel-tetrapyrrole coenzyme, Cofactor F430
Cofactor F430
F430 is the prosthetic group of the enzyme methyl coenzyme M reductase. It is found only in methanogenic Archaea. This enzyme catalyzes the release of methane in the final step of methanogenesis:-Corphin in context:...
, is present in the methyl coenzyme M
Coenzyme M
Coenzyme M is a coenzyme required for methyl-transfer reactions in the metabolism of methanogens. The coenzyme is an anion with the formula . It is named 2-mercaptoethanesulfonate and abbreviated HS–CoM. The cation is unimportant, but the sodium salt is most available...
reductase, which powers methanogen
Methanogen
Methanogens are microorganisms that produce methane as a metabolic byproduct in anoxic conditions. They are classified as archaea, a group quite distinct from bacteria...
ic archaea
Archaea
The Archaea are a group of single-celled microorganisms. A single individual or species from this domain is called an archaeon...
. One of the carbon monoxide dehydrogenase enzymes consists of an Fe-Ni-S cluster. Other nickel-containing enzymes include a rare bacterial class of superoxide dismutase
Superoxide dismutase
Superoxide dismutases are a class of enzymes that catalyze the dismutation of superoxide into oxygen and hydrogen peroxide. As such, they are an important antioxidant defense in nearly all cells exposed to oxygen...
and glyoxalase I enzymes in bacteria and several parasitic eukaryotic trypanosomal parasites
(this enzyme in higher organisms, including yeast and mammals, uses divalent zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
, Zn2+).
Toxicity
Exposure to nickel metal and soluble compounds should not exceed 0.05 mg/cm³ in nickel equivalents per 40-hour work-week. Nickel sulfide fume and dust are believed to be carcinogenCarcinogen
A carcinogen is any substance, radionuclide, or radiation that is an agent directly involved in causing cancer. This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes...
ic, and various other nickel compounds may be as well. Nickel carbonyl
Nickel carbonyl
Nickel carbonyl is the organonickel compound with the formula Ni4. This pale-yellow liquid is the principal carbonyl of nickel. It is an intermediate in the Mond process for the purification of nickel and a reagent in organometallic chemistry...
, [Ni(CO)4], is an extremely toxic gas. The toxicity of metal carbonyls is a function of both the toxicity of the metal as well as the carbonyl's ability to give off highly toxic carbon monoxide
Carbon monoxide
Carbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
gas, and this one is no exception; nickel carbonyl is also explosive in air.
Sensitized
Sensitization
Sensitization is an example of non-associative learning in which the progressive amplification of a response follows repeated administrations of a stimulus. An everyday example of this mechanism is the repeated tonic stimulation of peripheral nerves that will occur if a person rubs his arm...
individuals may show an allergy
Allergy
An Allergy is a hypersensitivity disorder of the immune system. Allergic reactions occur when a person's immune system reacts to normally harmless substances in the environment. A substance that causes a reaction is called an allergen. These reactions are acquired, predictable, and rapid...
to nickel, affecting their skin, also known as dermatitis
Dermatitis
-Etymology:Dermatitis derives from Greek derma "skin" + -itis "inflammation" and genetic disorder.-Terminology:There are several different types of dermatitis. The different kinds usually have in common an allergic reaction to specific allergens. The term may describe eczema, which is also called...
. Sensitivity to nickel may also be present in patients with pompholyx
Dyshidrosis
Dyshidrosis is a skin condition that is characterized by small blisters on the hands or feet...
. Nickel is an important cause of contact allergy, partly due to its use in jewellery intended for pierced ears. Nickel allergies affecting pierced ears are often marked by itchy, red skin. Many earrings are now made nickel-free due to this problem. The amount of nickel allowed in products that come into contact with human skin is regulated by the European Union
European Union
The European Union is an economic and political union of 27 independent member states which are located primarily in Europe. The EU traces its origins from the European Coal and Steel Community and the European Economic Community , formed by six countries in 1958...
. In 2002, researchers found amounts of nickel being emitted by 1 and 2 Euro coins far in excess of those standards. This is believed to be due to a galvanic
Galvanization
Galvanization is the process of applying a protective zinc coating to steel or iron, in order to prevent rusting. The term is derived from the name of Italian scientist Luigi Galvani....
reaction.
It was voted Allergen of the Year
Allergen of the Year
Allergen of the Year is an annual 'award' of dubious distinction voted upon by the American Contact Dermatitis Society. It is "designed to draw attention to allergens that are very common and/or under-recognized and merit more attention because they are causing significant allergic contact...
in 2008 by the American Contact Dermatitis Society.
Reports also showed that both the nickel-induced activation of hypoxia-inducible factor (HIF-1) and the up-regulation of hypoxia-inducible genes are due to depleted intracellular ascorbate levels. The addition of ascorbate to the culture medium increased the intracellular ascorbate level and reversed both the metal-induced stabilization of HIF-1- and HIF-1α-dependent gene expression.
See also
- :Category:Nickel alloys
- Nickel aluminideNickel aluminideNickel aluminide is an intermetallic material with properties similar to both a ceramic and a metal.There are three materials called nickel aluminide:* NiAl, CAS number 12003-78-0 * NiAl3, CAS number 12004-71-6* Ni3Al-Ni3Al:...
- Raney nickelRaney nickelRaney nickel is a solid catalyst composed of fine grains of a nickel-aluminium alloy, used in many industrial processes. It was developed in 1926 by American]] engineer Murray Raney as an alternative catalyst for the hydrogenation of vegetable oils in industrial processes...
- SuperalloySuperalloyA superalloy, or high-performance alloy, is an alloy that exhibits excellent mechanical strength and creep resistance at high temperatures, good surface stability, and corrosion and oxidation resistance. Superalloys typically have a matrix with an austenitic face-centered cubic crystal structure. ...
- Energy CatalyzerEnergy CatalyzerThe Energy Catalyzer is a supposed cold fusion or Low-Energy Nuclear Reaction heat source built by inventor Andrea Rossi, with support from physicist Sergio Focardi...
External links
- WebElements.com – Nickel
- An occupational hygiene assessment of dermal nickel exposures in primary production industries by GW Hughson. Institute of Occupational MedicineInstitute of Occupational MedicineThe Institute of Occupational Medicine was founded in 1969 by the National Coal Board as an independent charity. The IOM is a major independent centre of scientific excellence in the fields of occupational health and environmental health, occupational hygiene and occupational safety...
Research Report TM/04/05 - An occupational hygiene assessment of dermal nickel exposures in primary production and primary user industries. Phase 2 Report by GW Hughson. Institute of Occupational MedicineInstitute of Occupational MedicineThe Institute of Occupational Medicine was founded in 1969 by the National Coal Board as an independent charity. The IOM is a major independent centre of scientific excellence in the fields of occupational health and environmental health, occupational hygiene and occupational safety...
Research Report TM/05/06 - Norilsk Nickel, Norilsk, Russia
- Vale Nickel, Sudbury, Ontario, Canada (formerly known as INCO)
- Xstrata Nickel, Sudbury Operations (formerly known as Falconbridge)

