Alkaline fuel cell
Encyclopedia
Francis Thomas Bacon
Francis Thomas Bacon OBE FREng F.R.S. was an English engineer who developed the first practical hydrogen–oxygen fuel cell.- Life and works :...
fuel cell after its British inventor, is one of the most developed fuel cell
Fuel cell
A fuel cell is a device that converts the chemical energy from a fuel into electricity through a chemical reaction with oxygen or another oxidizing agent. Hydrogen is the most common fuel, but hydrocarbons such as natural gas and alcohols like methanol are sometimes used...
technologies. NASA
NASA
The National Aeronautics and Space Administration is the agency of the United States government that is responsible for the nation's civilian space program and for aeronautics and aerospace research...
has used alkaline fuel cells since the mid-1960s, in Apollo
Project Apollo
The Apollo program was the spaceflight effort carried out by the United States' National Aeronautics and Space Administration , that landed the first humans on Earth's Moon. Conceived during the Presidency of Dwight D. Eisenhower, Apollo began in earnest after President John F...
-series missions and on the Space Shuttle
Space Shuttle program
NASA's Space Shuttle program, officially called Space Transportation System , was the United States government's manned launch vehicle program from 1981 to 2011...
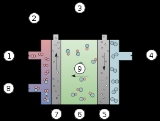
. AFCs consume hydrogen and pure oxygen producing potable water, heat, and electricity. They are among the most efficient fuel cells, having the potential to reach 70%.
Chemistry
The fuel cell produces power through a redox reaction between hydrogenHydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
and oxygen. At the anode
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
, hydrogen is oxidized according to the reaction:
 |
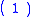 |
producing water and releasing two electrons. The electrons flow through an external circuit and return to the cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
, reducing oxygen in the reaction:
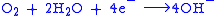 |
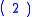 |
producing hydroxide
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
ions. The net reaction consumes one oxygen atom and two hydrogen atoms in the production of one water molecule. Electricity and heat are formed as by-products of this reaction.
Electrolyte
The two electrodes are separated by a porous matrix saturated with an aqueous alkaline solution, such as potassium hydroxide (KOH). Aqueous alkaline solutions do not reject carbon dioxide (CO2) so the fuel cell can become "poisoned" through the conversion of KOH to potassium carbonate (K2CO3). Because of this, alkaline fuel cells typically operate on pure oxygen, or at least purified air and would incorporate a 'scrubber' into the design to clean out as much of the carbon dioxide as is possible. Because the generation and storage requirements of oxygen make pure-oxygen AFCs expensive, there are few companies engaged in active development of the technology. There is, however, some debate in the research community over whether the poisoning is permanent or reversible. The main mechanisms of poisoning are blocking of the pores in the cathode with K2CO3, which is not reversible, and reduction in the ionic conductivity of the electrolyte, which may be reversible by returning the KOH to its original concentration. An alternate method involves simply replacing the KOH which returns the cell back to its original output.Basic Designs
Because of this poisoning effect, two main variants of AFCs exist: static electrolyte and flowing electrolyte. Static, or immobilized, electrolyte cells of the type used in the Apollo space craft and the Space Shuttle typically use an asbestos separator saturated in potassium hydroxide. Water production is managed by evaporation out the anode, as pictured above, which produces pure water that may be reclaimed for other uses. These fuel cells typically use platinum catalysts to achieve maximum volumetric and specific efficiencies.Flowing electrolyte designs use a more open matrix that allows the electrolyte to flow either between the electrodes (parallel to the electrodes) or through the electrodes in a transverse direction (the ASK-type or EloFlux fuel cell). In parallel-flow electrolyte designs, the water produced is retained in the electrolyte, and old electrolyte may be exchanged for fresh, in a manner analogous to an oil change in a car . In the case of "parallel flow" designs, greater space is required between electrodes to enable this flow, and this translates into an increase in cell resistance, decreasing power output compared to immobilized electrolyte designs. A further challenge for the technology is that it is not clear how severe is the problem of permanent blocking of the cathode by K2CO3, however, some published reports indicate thousands of hours of operation on air. These designs have used both platinum and non-noble metal catalysts, resulting in increased volumetric and specific efficiencies and increased cost.
The EloFlux design, with its transverse flow of electrolyte, has the advantage of low-cost construction and replaceable electrolyte, but so far has only been demonstrated using oxygen.
Further variations on the alkaline fuel cell include the metal hydride fuel cell
Metal hydride fuel cell
Metal hydride fuel cells are a subclass of alkaline fuel cells that are currently in the research and development phase. A notable feature is their ability to chemically bond and store hydrogen within the cell. This feature is shared with direct borohydride fuel cells, although the two differ in...
and the direct borohydride fuel cell
Direct borohydride fuel cell
Direct borohydride fuel cells are a subcategory of alkaline fuel cells which are directly fed by sodium borohydride or potassium borohydride as a fuel and either air/oxygen or hydrogen peroxide as the oxidant...
.
Commercial Prospects
AFCs are the cheapest of fuel cells to manufacture. The catalyst required for the electrodes can be any of a number of different chemicals that are inexpensive compared to those required for other types of fuel cells.The commercial prospects for AFCs lie largely with the recently developed bi-polar plate version of this technology, considerably superior in performance to earlier mono-plate versions.
The world's first Fuel Cell Ship HYDRA
Hydra (ship)
The Hydra is a 22 person hydrogen ship, power-assisted by an electric motor that gets its electricity from a fuel cell. The debut was in June 2000 on the Rhine near Bonn, Germany....
used an AFC system with 6.5 kW net output.
Another very interesting recent development (though not necessarily for high power applications) is the solid-state alkaline fuel cell, utilizing alkali anion exchange membrane
Alkali anion exchange membrane
An alkali anion exchange membrane is a semipermeable membrane generally made from ionomers and designed to conduct anions while being impermeable to gases such as oxygen or hydrogen...
s rather than a liquid.
See also
- Gas diffusion electrodeGas diffusion electrodeGas diffusion electrodes are electrodes with a conjunction of a solid, liquid and gaseous interface, and an electrical conducting catalyst supporting an electrochemical reaction between the liquid and the gaseous phase...
- Glossary of fuel cell termsGlossary of fuel cell termsThe Glossary of fuel cell terms lists the definitions of many terms used within the fuel cell industry. The terms in this fuel cell glossary may be used by fuel cell industry associations, in education material and fuel cell codes and standards to name but a few. –...
- HydrazineHydrazineHydrazine is an inorganic compound with the formula N2H4. It is a colourless flammable liquid with an ammonia-like odor. Hydrazine is highly toxic and dangerously unstable unless handled in solution. Approximately 260,000 tons are manufactured annually...
- Hydrogen technologiesHydrogen technologiesHydrogen technologies are technologies that relate to the production and use of hydrogen. Hydrogen technologies are applicable for many uses....
External links
Developers

