
Hydroxide
Encyclopedia


Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
OH−. It consists of an oxygen
Oxygen
Oxygen is the element with atomic number 8 and represented by the symbol O. Its name derives from the Greek roots ὀξύς and -γενής , because at the time of naming, it was mistakenly thought that all acids required oxygen in their composition...
and a hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
held together by a covalent bond
Covalent bond
A covalent bond is a form of chemical bonding that is characterized by the sharing of pairs of electrons between atoms. The stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding....
, and carrying a negative electric charge
Electric charge
Electric charge is a physical property of matter that causes it to experience a force when near other electrically charged matter. Electric charge comes in two types, called positive and negative. Two positively charged substances, or objects, experience a mutual repulsive force, as do two...
. It is an important but usually minor constituent of water. It functions as a base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
, as a ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
, a nucleophile
Nucleophile
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can act as nucleophiles. Because nucleophiles donate electrons, they are by definition Lewis bases.Nucleophilic describes the...
, and a catalyst . The hydroxide ion form salts, which dissociate
Dissociation (chemistry)
Dissociation in chemistry and biochemistry is a general process in which ionic compounds separate or split into smaller particles, ions, or radicals, usually in a reversible manner...
in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical
Commodity chemicals
Commodity chemicals is a group of chemicals including Alcohol, Amines, Betaines, Fatty alcohols, Acids, Wax, Oils and Petrolatum, Quats, Solvents, Surfactants....
. A hydroxide group attached to a strongly electropositive center may itself dissociate, liberating a hydrogen cation (H+), making the parent compound an acid.
In organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
, the hydroxide ion can act as a catalyst or as a nucleophilic reagent. An OH group, known as an hydroxyl group, is present in alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s, phenols
Phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of a hydroxyl group bonded directly to an aromatic hydrocarbon group...
, carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
s and related functional group
Functional group
In organic chemistry, functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. The same functional group will undergo the same or similar chemical reaction regardless of the size of the molecule it is a part of...
s.
Hydroxide ion
The hydroxide ion is a natural constituent of water, because of the self-ionizationSelf-ionization of water
The self-ionization of water is the chemical reaction in which a proton is transferred from one water molecule to another, in pure water or an aqueous solution, to create the two ions, hydronium, H3O+ and hydroxide, OH−...
reaction:
- H+ + OH− H2O
The equilibrium constant for this reaction, defined as
- Kw = [H+][OH−][H+] denotes the concentration of hydrogen cationsHydrogen ionHydrogen ion is recommended by IUPAC as a general term for all ions of hydrogen and its isotopes.Depending on the charge of the ion, two different classes can be distinguished: positively charged ions and negatively charged ions....
and [OH−] the concentration of hydroxide ions
has a value close to 10−14 at 25 °C, so the concentration
Concentration
In chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
of hydroxide ions in pure water is close to 10−7 mol dm−3, in order to satisfy the equal charge constraint. The pH
PH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
of a solution is equal to the decimal cologarithm
Cologarithm
In mathematics, the base-b cologarithm, sometimes shortened to colog, of a number is the base-b logarithm of the reciprocal of the number...
of the hydrogen cation concentration;Strictly speaking pH is the cologarithm of the hydrogen cation activity
Activity (chemistry)
In chemical thermodynamics, activity is a measure of the “effective concentration” of a species in a mixture, meaning that the species' chemical potential depends on the activity of a real solution in the same way that it would depend on concentration for an ideal solution.By convention, activity...
the pH of pure water is close to 7 at ambient temperatures. The concentration of hydroxide ions can be expressed in terms of pOH, which is close to 14 – pH,p(OH) signifies the minus the logarithm to base 10 of {OH−}, alternatively the logarithm of 1/{OH−} so pOH of pure water is also close to 7. Addition of a base to water will reduce the hydrogen cation concentration and therefore increase the hydroxide ion concentration (increase pH, decrease pOH) even if the base does not itself contain hydroxide. For example, ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
solutions have a pH greater than 7 due to the reaction NH3 + H+ NH4+, which results in a decrease in hydrogen cation concentration and an increase in hydroxide ion concentration. pOH can be kept at a nearly constant value with various buffer solution
Buffer solution
A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. It has the property that the pH of the solution changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a...
s.
In aqueous solution
Aqueous solution
An aqueous solution is a solution in which the solvent is water. It is usually shown in chemical equations by appending aq to the relevant formula, such as NaCl. The word aqueous means pertaining to, related to, similar to, or dissolved in water...
the hydroxide ion is a base
Base (chemistry)
For the term in genetics, see base A base in chemistry is a substance that can accept hydrogen ions or more generally, donate electron pairs. A soluble base is referred to as an alkali if it contains and releases hydroxide ions quantitatively...
in the Brønsted–Lowry sense as it can accept a protonIn this context proton is the term used for a solvated hydrogen cation from a Brønsted–Lowry acid to form a water molecule. It can also act as a Lewis base by donating a pair of electrons to a Lewis acid. In aqueous solution both hydrogen and hydroxide ions are strongly solvated, with hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
s between oxygen and hydrogen atoms. Indeed, the bihydroxide ion, H3O2−, has been characterized in the solid state. This compound is centrosymmetric and has a very short hydrogen bond (114.5 pm) that is similar to the length in the bifluoride ion, HF2− (114 pm). In aqueous solution the hydroxide ion forms strong hydrogen bonds with water molecules. A consequence of this is that concentrated solutions of sodium hydroxide have high viscosity
Viscosity
Viscosity is a measure of the resistance of a fluid which is being deformed by either shear or tensile stress. In everyday terms , viscosity is "thickness" or "internal friction". Thus, water is "thin", having a lower viscosity, while honey is "thick", having a higher viscosity...
due to the formation of an extended network of hydrogen bonds as in hydrogen fluoride
Hydrogen fluoride
Hydrogen fluoride is a chemical compound with the formula HF. This colorless gas is the principal industrial source of fluorine, often in the aqueous form as hydrofluoric acid, and thus is the precursor to many important compounds including pharmaceuticals and polymers . HF is widely used in the...
solutions.
In solution, exposed to air, the hydroxide ion reacts rapidly with atmospheric carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
, acting as an acid, to form, initially, the bicarbonate
Bicarbonate
In inorganic chemistry, bicarbonate is an intermediate form in the deprotonation of carbonic acid...
ion.
- OH− + CO2 HCO3−
The equilibrium constant for this reaction can be specified either as a reaction with dissolved carbon dioxide or as a reaction with carbon dioxide gas (see carbonic acid
Carbonic acid
Carbonic acid is the inorganic compound with the formula H2CO3 . It is also a name sometimes given to solutions of carbon dioxide in water, because such solutions contain small amounts of H2CO3. Carbonic acid forms two kinds of salts, the carbonates and the bicarbonates...
for values and details). At neutral or acid pH, the reaction is slow, but is catalyzed by the enzyme
Enzyme
Enzymes are proteins that catalyze chemical reactions. In enzymatic reactions, the molecules at the beginning of the process, called substrates, are converted into different molecules, called products. Almost all chemical reactions in a biological cell need enzymes in order to occur at rates...
carbonic anhydrase
Carbonic anhydrase
The carbonic anhydrases form a family of enzymes that catalyze the rapid interconversion of carbon dioxide and water to bicarbonate and protons , a reversible reaction that occurs rather slowly in the absence of a catalyst...
, which effectively creates hydroxide ions at the active site.
Solutions containing the hydroxide ion attack glass
Glass
Glass is an amorphous solid material. Glasses are typically brittle and optically transparent.The most familiar type of glass, used for centuries in windows and drinking vessels, is soda-lime glass, composed of about 75% silica plus Na2O, CaO, and several minor additives...
. In this case, the silicate
Silicate
A silicate is a compound containing a silicon bearing anion. The great majority of silicates are oxides, but hexafluorosilicate and other anions are also included. This article focuses mainly on the Si-O anions. Silicates comprise the majority of the earth's crust, as well as the other...
s in glass are acting as acids. Basic hydroxides, whether solids or in solution, are stored in air-tight plastic containers.
The hydroxide ion can function as a typical electron-pair donor ligand
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
, forming such complexes as [Al(OH)4]–. It is also often found in mixed-ligand complexes of the type [MLx(OH)y]z+, where L is a ligand. The hydroxide ion often serves as a bridging ligand
Bridging ligand
A bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually restricted to small ligands such as pseudohalides or to ligands that are...
, donating one pair of electrons to each of the atoms being bridged. As illustrated by [Pb2(OH)]3+, metal hydroxides are often written in a simplified format. It can even act as a 3 electron-pair donor, as in the tetramer [PtMe3OH]4).
When bound to a strongly electron-withdrawing metal centre, hydroxide ligands tend to ionise
Dissociation (chemistry)
Dissociation in chemistry and biochemistry is a general process in which ionic compounds separate or split into smaller particles, ions, or radicals, usually in a reversible manner...
s into oxide ligands. For example, the bichromate ion, written as [HCrO4]–, dissociates according to
- [O3CrO-H]– [CrO4]2– + H+
with a pKa of about 5.9.
Vibrational spectra
The infrared spectra of compounds containing the OH group have strong absorption bands in the region centered around 3500 cm−1. The high frequency is a consequenceMolecular vibration
A molecular vibration occurs when atoms in a molecule are in periodic motion while the molecule as a whole has constant translational and rotational motion...
of the small mass of the hydrogen atom as compared to the mass of the oxygen atom and this makes detection of hydroxyl groups by infrared spectroscopy relatively easy A band due to an OH group tends to be sharp. However, the band width
Spectral linewidth
The spectral linewidth characterizes the width of a spectral line, such as in the electromagnetic emission spectrum of an atom, or the frequency spectrum of an acoustic or electronic system...
increases when the OH group is involved in hydrogen bonding. A water molecule has an HOH bending mode at about 1600 cm−1, so the absence of this band can be used to distinguish an OH group from a water molecule.
When the OH group is bound to a metal ion in a coordination complex, an M-OH bending mode can be observed. For example, in [Sn(OH)6]2– it occurs at 1065 cm−1. The bending mode for a bridging hydroxide tends to be at a lower frequency as in [(bipyridine
Bipyridine
Bipyridines are a family of chemical compounds with the formula 2, which are formed by the coupling of two pyridine rings. Six isomers of bipyridine exist, but two isomers are prominent: 2,2'-bipyridine is a popular ligand in coordination chemistry and 4,4'-bipyridine is a precursor to the...
)Cu(OH)2Cu(bipyridine
Bipyridine
Bipyridines are a family of chemical compounds with the formula 2, which are formed by the coupling of two pyridine rings. Six isomers of bipyridine exist, but two isomers are prominent: 2,2'-bipyridine is a popular ligand in coordination chemistry and 4,4'-bipyridine is a precursor to the...
)]2+ (955 cm−1). M-OH stretching vibrations occur below about 600 cm−1. For example, the tetrahedral ion [Zn(OH)4]2– has bands at 470 cm−1 (Raman
Raman spectroscopy
Raman spectroscopy is a spectroscopic technique used to study vibrational, rotational, and other low-frequency modes in a system.It relies on inelastic scattering, or Raman scattering, of monochromatic light, usually from a laser in the visible, near infrared, or near ultraviolet range...
-active, polarized) and 420 cm−1 (infrared). The same ion has an (OH)Zn(OH) bending vibration at 300 cm−1.
Applications
Sodium hydroxide solutions, also known as lye and caustic soda, are used in the manufacture of pulpWood pulp
Pulp is a lignocellulosic fibrous material prepared by chemically or mechanically separating cellulose fibres from wood, fibre crops or waste paper. Wood pulp is the most common raw material in papermaking.-History:...
and paper
Paper
Paper is a thin material mainly used for writing upon, printing upon, drawing or for packaging. It is produced by pressing together moist fibers, typically cellulose pulp derived from wood, rags or grasses, and drying them into flexible sheets....
, textile
Textile
A textile or cloth is a flexible woven material consisting of a network of natural or artificial fibres often referred to as thread or yarn. Yarn is produced by spinning raw fibres of wool, flax, cotton, or other material to produce long strands...
s, drinking water
Drinking water
Drinking water or potable water is water pure enough to be consumed or used with low risk of immediate or long term harm. In most developed countries, the water supplied to households, commerce and industry is all of drinking water standard, even though only a very small proportion is actually...
, soap
Soap
In chemistry, soap is a salt of a fatty acid.IUPAC. "" Compendium of Chemical Terminology, 2nd ed. . Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford . XML on-line corrected version: created by M. Nic, J. Jirat, B. Kosata; updates compiled by A. Jenkins. ISBN...
s and detergent
Detergent
A detergent is a surfactant or a mixture of surfactants with "cleaning properties in dilute solutions." In common usage, "detergent" refers to alkylbenzenesulfonates, a family of compounds that are similar to soap but are less affected by hard water...
s, and as a drain cleaner
Drain cleaner
A drain cleaner is a consumer product or device that unblocks sewer pipes or helps to prevent the occurrence of clogged drains; the term may also refer to the individual who performs the activity...
. Worldwide production in 2004 was approximately 60 million tonne
Tonne
The tonne, known as the metric ton in the US , often put pleonastically as "metric tonne" to avoid confusion with ton, is a metric system unit of mass equal to 1000 kilograms. The tonne is not an International System of Units unit, but is accepted for use with the SI...
s. The principal method of manufacture is the chlor-alkali process.
Solutions containing the hydroxide ion are generated when a salt of a weak acid
Weak acid
A weak acid is an acid that dissociates incompletely. It does not release all of its hydrogens in a solution, donating only a partial amount of its protons to the solution...
is dissolved in water. Sodium carbonate
Sodium carbonate
Sodium carbonate , Na2CO3 is a sodium salt of carbonic acid. It most commonly occurs as a crystalline heptahydrate, which readily effloresces to form a white powder, the monohydrate. Sodium carbonate is domestically well-known for its everyday use as a water softener. It can be extracted from the...
is used as an alkali, for example, by virtue of the hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
reaction
- CO32– + H2O HCO3– + OH–; (pKa2Acid dissociation constantAn acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
= 10.33 at 25 °C and zero ionic strengthIonic strengthThe ionic strength of a solution is a measure of the concentration of ions in that solution. Ionic compounds, when dissolved in water, dissociate into ions. The total electrolyte concentration in solution will affect important properties such as the dissociation or the solubility of different salts...
)
Although the base strength of sodium carbonate solutions is lower than a concentrated sodium hydroxide solution, it has the advantage of being a solid. It is also manufactured on a vast scale (42 million tonnes in 2005) by the Solvay process
Solvay process
The Solvay process, also referred to as the ammonia-soda process, is the major industrial process for the production of soda ash . The ammonia-soda process was developed into its modern form by Ernest Solvay during the 1860s...
. An example of the use of sodium carbonate as an alkali is when washing soda (another name for sodium carbonate) acts on insoluble esters, such as triglycerides, commonly known as fats, to hydrolyze them and make them soluble.
Bauxite
Bauxite
Bauxite is an aluminium ore and is the main source of aluminium. This form of rock consists mostly of the minerals gibbsite Al3, boehmite γ-AlO, and diaspore α-AlO, in a mixture with the two iron oxides goethite and hematite, the clay mineral kaolinite, and small amounts of anatase TiO2...
, a basic hydroxide of aluminium
Aluminium
Aluminium or aluminum is a silvery white member of the boron group of chemical elements. It has the symbol Al, and its atomic number is 13. It is not soluble in water under normal circumstances....
, is the principal ore from which the metal is manufactured. Similarly, goethite
Goethite
Goethite , named after the German polymath Johann Wolfgang von Goethe, is an iron bearing oxide mineral found in soil and other low-temperature environments. Goethite has been well known since prehistoric times for its use as a pigment. Evidence has been found of its use in paint pigment samples...
(α-FeO(OH)) and lepidocrocite
Lepidocrocite
Lepidocrocite , also called esmeraldite or hydrohematite, is an iron oxide-hydroxide mineral. Lepidocrocite has an orthorhombic crystal structure, a hardness of 5, specific gravity of 4, a submetallic luster and a yellow-brown streak. It is red to reddish brown and forms when iron-containing...
(γ-FeO(OH)), basic hydroxides of iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
, are among the principal ores used for the manufacture of metallic iron. Numerous other uses can be found in the articles on individual hydroxides.
Alkali metals
Aside from NaOH and KOH, which enjoy very large scale applications, the hydroxides of the other alkali metals also are useful. Lithium hydroxideLithium hydroxide
Lithium hydroxide is an inorganic compound with the formula LiOH. It is a white hygroscopic crystalline material. It is soluble in water and slightly soluble in ethanol...
is a weak base, with pKb
Acid dissociation constant
An acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
of 0.2. Lithium hydroxide is used in breathing gas
Breathing gas
Breathing gas is a mixture of gaseous chemical elements and compounds used for respiration.Air is the most common and only natural breathing gas...
purification systems for spacecraft
Spacecraft
A spacecraft or spaceship is a craft or machine designed for spaceflight. Spacecraft are used for a variety of purposes, including communications, earth observation, meteorology, navigation, planetary exploration and transportation of humans and cargo....
, submarine
Submarine
A submarine is a watercraft capable of independent operation below the surface of the water. It differs from a submersible, which has more limited underwater capability...
s, and rebreather
Rebreather
A rebreather is a type of breathing set that provides a breathing gas containing oxygen and recycled exhaled gas. This recycling reduces the volume of breathing gas used, making a rebreather lighter and more compact than an open-circuit breathing set for the same duration in environments where...
s to remove carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
from exhaled gas.
- 2 LiOH + CO2 → Li2CO3 + H2O
The hydroxide of lithium is preferred to that of sodium because of its lower mass. Sodium hydroxide, potassium hydroxide
Potassium hydroxide
Potassium hydroxide is an inorganic compound with the formula KOH, commonly called caustic potash.Along with sodium hydroxide , this colorless solid is a prototypical strong base. It has many industrial and niche applications. Most applications exploit its reactivity toward acids and its corrosive...
and the hydroxides of the other alkali metals are strong bases.
Alkaline earth metals
Beryllium hydroxideBeryllium hydroxide
Beryllium hydroxide, Be2 is an amphoteric hydroxide, dissolving in both acids and alkalis. Industrially it is produced as a by-product in the extraction of beryllium metal from the ores, beryl and bertrandite. When alkali is added to beryllium salt solutions the α-form is formed. If this left to...
, Be(OH)2, is amphoteric. The hydroxide itself is insoluble in water, with a solubility product, log K*sp, of −11.7. Addition of acid gives soluble hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
products, including the trimeric ion [Be3(OH)3(H2O)6]3+, which has OH groups bridging between pairs of beryllium ions making a 6-membered ring. At very low pH the aqua ion
Metal ions in aqueous solution
A metal ion in aqueous solution is a cation, dissolved in water, of chemical formula [Mn]z+. The solvation number, n, determined by a variety of experimental methods is 4 for Li+ and Be2+ and 6 for elements in rows 3 and 4 of the periodic table. Lanthanide and actinide aqua ions have solvation...
[Be(H2O)4]2+ is formed. Addition of hydroxide to Be(OH)2 gives the soluble tetrahydroxo anion [Be(OH)4]2–.
The solubility in water of the other hydroxides in this group increases with increasing atomic number
Atomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
. Magnesium hydroxide
Magnesium hydroxide
Magnesium hydroxide is an inorganic compound with the chemical formula Mg2. As a suspension in water, it is often called milk of magnesia because of its milk-like appearance. The solid mineral form of magnesium hydroxide is known as brucite....
, Mg(OH)2, is a weak base but calcium hydroxide
Calcium hydroxide
Calcium hydroxide, traditionally called slaked lime, is an inorganic compound with the chemical formula Ca2. It is a colourless crystal or white powder and is obtained when calcium oxide is mixed, or "slaked" with water. It has many names including hydrated lime, builders lime, slack lime, cal, or...
is a strong base as are the hydroxides of the heavier alkaline earths, strontium hydroxide
Strontium hydroxide
Strontium hydroxide, Sr2, is a caustic alkali composed of one strontium ion and two hydroxide ions. It is synthesized by combining a strontium salt with a strong base...
and barium hydroxide
Barium hydroxide
Barium hydroxide is the chemical compound with the formula Ba2. Also known as baryta, it is one of the principal compounds of barium. The white granular monohydrate is the usual commercial form.-Preparation:...
. A solution/suspension of calcium hydroxide is known as lime water
Lime water
Limewater is the common name for saturated calcium hydroxide solution. It is sparsely soluble. Its chemical formula is Ca2. Since calcium hydroxide is only sparsely soluble, i.e. ca. 1.5 g per liter at 25 °C, there is no visible distinction to clear water. Attentive observers will notice a slightly...
and can be used to test for the weak acid
Weak acid
A weak acid is an acid that dissociates incompletely. It does not release all of its hydrogens in a solution, donating only a partial amount of its protons to the solution...
carbon dioxide. The reaction Ca(OH)2 + CO2 Ca2+ + [HCO3]– + OH– illustrates the strong basicity of calcium hydroxide. Soda lime
Soda lime
Soda lime is a mixture of chemicals, used in granular form in closed breathing environments, such as general anaesthesia, submarines, rebreathers and recompression chambers, to remove carbon dioxide from breathing gases to prevent CO2 retention and carbon dioxide poisoning.It is made by treating...
, which is a mixture of NaOH and Ca(OH)2 is used as a CO2 absorbent.
Boron group elements
The simplest hydroxide of boron, B(OH)3, known as boric acidBoric acid
Boric acid, also called hydrogen borate or boracic acid or orthoboric acid or acidum boricum, is a weak acid of boron often used as an antiseptic, insecticide, flame retardant, as a neutron absorber, and as a precursor of other chemical compounds. It exists in the form of colorless crystals or a...
, is an acid. Unlike the hydroxides of the alkali and alkaline earth hydroxides, it does not dissociate in aqueous solution. Instead, it reacts with water molecules acting as a Lewis acid, releasing protons.
- B(OH)3 + H2O [B(OH)4]–TetrahydroxyborateTetrahydroxyborate, [H4BO4]− or B, is a boron oxoanion with a tetrahedral geometry. It is isoelectronic with the hypothetical compound orthocarbonic acid.B is formed by the addition of hydroxide, OH−, to boric acid, B3:...
+ H+
A variety of oxyanion
Oxyanion
An oxyanion or oxoanion is a chemical compound with the generic formula AxOyz− . Oxoanions are formed by a large majority of the chemical elements. The formulae of simple oxoanions are determined by the octet rule...
s of boron are known, which, in the protonated form, contain hydroxide groups.
Aluminium hydroxide, Al(OH)3 is amphoteric and dissolves in alkaline solution.
- Al(OH)3 (solid) + OH- (aq) [Al(OH)4]- (aq)
In the Bayer process
Bayer process
The Bayer process is the principal industrial means of refining bauxite to produce alumina .Bauxite, the most important ore of aluminium, contains only 30–54% alumina, Al2O3, the rest being a mixture of silica, various iron oxides, and titanium dioxide. The alumina must be purified before it can...
for the production of pure aluminium oxide from bauxite
Bauxite
Bauxite is an aluminium ore and is the main source of aluminium. This form of rock consists mostly of the minerals gibbsite Al3, boehmite γ-AlO, and diaspore α-AlO, in a mixture with the two iron oxides goethite and hematite, the clay mineral kaolinite, and small amounts of anatase TiO2...
minerals this equilibrium is manipulated by careful control of temperature and alkali concentration. In the first phase, aluminium dissolves in hot alkaline solution as [Al(OH)4]- but other hydroxides usually present in the mineral, such as iron hydroxides, do not dissolve because they are not amphoteric. After removal of the insolubles, the so-called red mud
Red mud
Red mud is a solid waste product of the Bayer process, the principal industrial means of refining bauxite in order to provide alumina as raw material for the electrolysis of aluminium by the Hall–Héroult process. A typical plant produces one to two times as much red mud as alumina...
, pure aluminium hydroxide is made to precipitate by reducing the temperature and adding water to the extract, which, by diluting the alkali, lowers the pH of the solution. Basic aluminium hydroxide, AlO(OH), which may be present in bauxite is also amphoteric.
In mildly acidic solutions the hydroxo complexes formed by aluminium are somewhat different from those of boron, reflecting the greater size of Al(III) vs. B(III). The concentration of the species [Al13(OH)32]7+ is very dependent on the total aluminium concentration. Various other hydroxo complexes are found in crystalline compounds. Perhaps the most important is the basic hydroxide, AlO(OH), a polymeric material known by the names of the mineral forms boehmite
Boehmite
Boehmite or Böhmite is an aluminium oxide hydroxide mineral, a component of the aluminium ore bauxite. It is dimorphous with diaspore. It crystallizes in the orthorhombic dipyramidal system and is typically massive in habit. It is white with tints of yellow, green, brown or red due to impurities...
or diaspore
Diaspore
Diaspore is a native aluminium oxide hydroxide, α-AlO, crystallizing in the orthorhombic system and isomorphous with goethite. It occurs sometimes as flattened crystals, but usually as lamellar or scaly masses, the flattened surface being a direction of perfect cleavage on which the lustre is...
, depending on crystal structure. Gallium hydroxide, indium hydroxide and thallium(III) hydroxide
Thallium(III) hydroxide
Thallium hydroxide, Tl3, is a hydroxide of thallium. It is a white solid.Thallium hydroxide is a very weak base; it is changed to thallium ion, Tl3+, only in strongly acid conditions....
s are also amphoteric. Thallium(I) hydroxide
Thallium(I) hydroxide
Thallium hydroxide, also called thallous hydroxide, TlOH, is a hydroxide of thallium, with thallium in oxidation state +1. Thallous hydroxide is a strong base; it is changed to thallous ion, Tl+, except in strongly basic conditions.-References:...
is a strong base.
Carbon group elements
Carbon forms no simple hydroxides. The hypothetical compound C(OH)4 is unstable in aqueous solution:- C(OH)4 → HCO3– + H3O+
- HCO3– + H+ H2CO3
Carbon dioxide
Carbon dioxide
Carbon dioxide is a naturally occurring chemical compound composed of two oxygen atoms covalently bonded to a single carbon atom...
is also known as carbonic anhydride, meaning that it forms by dehydration of carbonic acid, H2CO3 (OC(OH)2).
Silicic acid
Silicic acid
Silicic acid is a general name for a family of chemical compounds of the element silicon, hydrogen, and oxygen, with the general formula [SiOx4-2x]n...
is the name given to a variety of compounds with a generic formula [SiOx(OH)4-2x]n. Orthosilicic acid have been identified in very dilute aqueous solution. It is a weak acid with pKa1 = 9.84, pKa2 = 13.2 at 25 °C. It is usually written as H4SiO4 but the formula SiO2(OH)2 is generally accepted . Other silicic acids such as metasilicic acid (H2SiO3), disilicic acid (H2Si2O5), and pyrosilicic acid (H6Si2O7) have been characterized. These acids also have hydroxide groups attached to the silicon; the formulas suggest that these acid are protonated forms of polyoxyanion
Oxyanion
An oxyanion or oxoanion is a chemical compound with the generic formula AxOyz− . Oxoanions are formed by a large majority of the chemical elements. The formulae of simple oxoanions are determined by the octet rule...
s.
Few hydroxo complexes of germanium
Germanium
Germanium is a chemical element with the symbol Ge and atomic number 32. It is a lustrous, hard, grayish-white metalloid in the carbon group, chemically similar to its group neighbors tin and silicon. The isolated element is a semiconductor, with an appearance most similar to elemental silicon....
have been characterized. Tin(II) hydroxide, Sn(OH)2, was prepared in anhydrous media. When tin(II) oxide
Tin(II) oxide
Tin oxide is a compound of tin and oxygen where tin has the oxidation state of +2. There are two forms, a stable blue-black form and a metastable red form.-Preparation and reactions:...
is treated with alkali the pyramidal hydroxo complex Sn(OH)3– is formed. When solutions containing this ion are acidified the ion [Sn3(OH)4]2+ is formed together with some basic hydroxo complexes. The structure of [Sn3(OH)4]2+ has a triangle of tin atoms connected by bridging hydroxide groups. Tin(IV) hydroxide is unknown but can be regarded as the hypothetical acid from which stannates, with a formula [Sn(OH)6]2–, are derived by reaction with the (Lewis) basic hydroxide ion.
Hydrolysis of Pb2+ in aqueous solution is accompanied by the formation of various hydroxo-containing complexes, some of which are insoluble. The basic hydroxo complex [Pb6O(OH)6]4+ is a cluster of six lead centres with metal-metal bonds surrounding a central oxide ion. The six hydroxide groups lie on the faces of the two external Pb4 tetrahedra. In strongly alkaline solutions soluble plumbate
Plumbate
In chemistry, a plumbate is a salt having one of the several lead-containing oxoanions. Although the term plumbate can refer either to plumbate or plumbate, it traditionally refers specifically to plumbate, whereas plumbate is referred to as plumbite.Plumbates are formed by the reaction of lead...
ions are formed, including [Pb(OH)6]2−.
Other main-group elements
 |
||||
| Phosphorous acid Phosphorous acid Phosphorous acid is the compound described by the formula H3PO3. This acid is diprotic , not triprotic as might be suggested by this formula. Phosphorous acid is as an intermediate in the preparation of other phosphorus compounds.-Nomenclature and tautomerism:H3PO3 is more clearly described with... |
Phosphoric acid Phosphoric acid Phosphoric acid, also known as orthophosphoric acid or phosphoric acid, is a mineral acid having the chemical formula H3PO4. Orthophosphoric acid molecules can combine with themselves to form a variety of compounds which are also referred to as phosphoric acids, but in a more general way... |
Sulfuric acid Sulfuric acid Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates... |
Telluric acid Telluric acid Telluric acid is a chemical compound with the formula Te6. It is a white solid made up of octahedral Te6 molecules which persist in aqueous solution... |
orthoperiodic acid Periodic acid Periodic acid, or iodic acid is an oxoacid of iodine having chemical formula HIO4 or H5IO6.In dilute aqueous solution, periodic acid exists as discrete hydronium and metaperiodate ions. When more concentrated, orthoperiodic acid, H5IO6, is formed; this dissociates into hydronium and... |
In the higher oxidation states of the elements in groups 5, 6 and 7 there are oxoacids in which the central atom is attached to oxide ions and hydroxide ions. Examples include phosphoric acid
Phosphoric acid
Phosphoric acid, also known as orthophosphoric acid or phosphoric acid, is a mineral acid having the chemical formula H3PO4. Orthophosphoric acid molecules can combine with themselves to form a variety of compounds which are also referred to as phosphoric acids, but in a more general way...
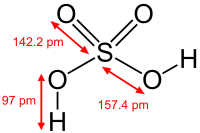
, H3PO4 and sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
, H2SO4. In these compounds one or more hydroxide groups can dissociate
Dissociation (chemistry)
Dissociation in chemistry and biochemistry is a general process in which ionic compounds separate or split into smaller particles, ions, or radicals, usually in a reversible manner...
with the liberation of hydrogen cations as in a standard Brønsted–Lowry acid. Many oxoacids of sulfur are known and all feature OH groups that can dissociate.
Telluric acid
Telluric acid
Telluric acid is a chemical compound with the formula Te6. It is a white solid made up of octahedral Te6 molecules which persist in aqueous solution...
is often written with the formula H2TeO4·2H2O but is better described structurally as Te(OH)6.
Ortho-periodic acidThe name is not derived from "period", but from "iodine": per-iodic acid (compare iodic acid
Iodic acid
Iodic acid, HIO3, can be obtained as a white solid. It dissolves in water very well, but it also exists in the pure state, as opposed to chloric acid or bromic acid. Iodic acid contains iodine in the oxidation state +5 and it is one of the most stable oxo-acids of the halogens in its pure state....
, perchloric acid
Perchloric acid
Perchloric acid is the inorganic compound with the formula HClO4. Usually encountered as an aqueous solution, this colourless compound is a strong acid comparable in strength to sulfuric and nitric acids. It is a powerful oxidizer, but its aqueous solutions up to appr. 70% are remarkably inert,...
), and it is thus pronounced per-iodic ˌpɜr.aɪˈɒdɨk , and not as ˌpɪərɪˈɒdɨk . can lose all its protons, eventually forming the periodate ion, [IO4]–. It can also be protonated in strongly acidic conditions to give the octahedral ion [I(OH)6]+, completing the isoelectronic series, [E(OH)6]z, E = Sn, Sb, Te, I; z = -2, −1, 0, +1. Other acids of iodine(VII) that contain hydroxide groups are known, in particular in salts such as the mesoperiodate ion that occurs in K4[I2O8(OH)2]·8H2O.
As is common outside of the alkali metals, hydroxides of the elements in lower oxidation states are complicated. For example, phosphorous acid
Phosphorous acid
Phosphorous acid is the compound described by the formula H3PO3. This acid is diprotic , not triprotic as might be suggested by this formula. Phosphorous acid is as an intermediate in the preparation of other phosphorus compounds.-Nomenclature and tautomerism:H3PO3 is more clearly described with...
, H3PO3, predominantly has the structure OP(H)(OH)2, in equilibrium with a small amount of P(OH)3.
The oxoacids of chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
, bromine
Bromine
Bromine ") is a chemical element with the symbol Br, an atomic number of 35, and an atomic mass of 79.904. It is in the halogen element group. The element was isolated independently by two chemists, Carl Jacob Löwig and Antoine Jerome Balard, in 1825–1826...
and iodine
Iodine
Iodine is a chemical element with the symbol I and atomic number 53. The name is pronounced , , or . The name is from the , meaning violet or purple, due to the color of elemental iodine vapor....
have the formula O(n–1)/2A(OH) where n is the oxidation number
Oxidation number
In coordination chemistry, the oxidation number of a central atom in a coordination compound is the charge that it would have if all the ligands were removed along with the electron pairs that were shared with the central atom. Oxidation numbers are often confused with oxidation states.The...
, +1, +3 or +5, and A = Cl, Br or I. The only oxoacid of fluorine
Fluorine
Fluorine is the chemical element with atomic number 9, represented by the symbol F. It is the lightest element of the halogen column of the periodic table and has a single stable isotope, fluorine-19. At standard pressure and temperature, fluorine is a pale yellow gas composed of diatomic...
is F(OH). When these acids are neutralized the hydrogen atom is removed from the hydroxide group.
Transition and post-transition metals
The hydroxides of the transition metalTransition metal
The term transition metal has two possible meanings:*The IUPAC definition states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Group 12 elements are not transition metals in this definition.*Some...
s and post-transition metal
Post-transition metal
In chemistry, the term post-transition metal is used to describe the category of metallic elements to the right of the transition elements on the periodic table...
s usually have the metal in the +2 (M = Mn, Fe, Co, Ni, Cu, Zn) or +3 (M = Fe, Ru, Rh, Ir) oxidation state. None are soluble in water, and many are poorly defined. Hydroxides of metals in the +1 oxidation state are also poorly defned or unstable. For example, silver hydroxide, Ag(OH), decomposes spontaneously to the oxide (Ag2O). Copper(I) and gold(I) hydroxides are also unstable, though stable adducts of CuOH and AuOH are known. The polymeric compounds M(OH)2 and M(OH)3 are in general prepared by increasing the pH of an aqueous solutions of the corresponding metal cations until the hydroxide precipitates out of solution. On the converse, the hydroxides dissolve in acidic solution. Zinc hydroxide
Zinc hydroxide
Zinc hydroxide Zn2 is an inorganic chemical compound. It also occurs naturally as 3 rare minerals: wülfingite , ashoverite and sweetite ....
, Zn(OH)2, is amphoteric, forming the zincate ion, Zn(OH)42– in strongly alkaline solution.
Numerous mixed ligand complexes of these metals with the hydroxide ion exist, in fact these are in general better defined than the simpler derivatives. Many can be made by causing dissociation of a co-ordinated water molecule.
- LnM(OH2) + B LnM(OH) + BH+ (L = ligand, B = base)
Vanadic acid, H3VO4, shows similarities with phosphoric acid, H3PO4, though it has a much more complex oxoanion
Vanadate
In chemistry, a vanadate is a compound containing an oxoanion of vanadium generally in its highest oxidation state of +5. The simplest vanadate ion is the tetrahedral, orthovanadate, VO43− anion, which is present in e.g. sodium orthovanadate and in solutions of V2O5 in strong base...
chemistry. Chromic acid
Chromic acid
The term chromic acid is usually used for a mixture made by adding concentrated sulfuric acid to a dichromate, which may contain a variety of compounds, including solid chromium trioxide. This kind of chromic acid may be used as a cleaning mixture for glass. Chromic acid may also refer to the...
, H2CrO4, has similarities with sulfuric acid, H2SO4; for example, both form acid salt
Acid salt
Acid salt is a somewhat obscure term for a class of salts formed by the partial neutralization of diprotic or polyprotic acids. Because the parent acid is only partially neutralized, one or more replaceable hydrogen atoms remain. Typical acid salts have one or more alkali metal ions as well as one...
s, A+[HMO4]–. Some metals, e.g. V, Cr, Nb, Ta, Mo, W, tend to exist in high oxidation states. Rather than forming hydroxides in aqueous solution, they convert to oxo clusters by the process of olation
Olation
In inorganic chemistry, olation is the process by which metal ions form polymeric oxides in aqueous solution. The phenomenon is important to understand the relationship between metal ions in aqueous solution and metal oxides, which are represented by many minerals.At low pH, many metal ions exist...
, forming polyoxometalate
Polyoxometalate
In chemistry, a polyoxometalate is a polyatomic ion, usually an anion, that consists of three or more transition metal oxyanions linked together by shared oxygen atoms to form a large, closed 3-dimensional framework....
s.
Basic salts containing hydroxide
In some cases the products of partial hydrolysis of metal ion, described above, can be found in crystalline compounds. A striking example is found with zirconiumZirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name of zirconium is taken from the mineral zircon. Its atomic mass is 91.224. It is a lustrous, grey-white, strong transition metal that resembles titanium...
(IV). Because of the high oxidation state, salts of Zr4+ are extensively hydrolyzed in water even at low pH. The compound originally formulated as ZrOCl2·8H2O was found to be the chloride salt of a tetrameric cation, [Zr4(OH)8(H2O)16]8+ in which there is a square of Zr4+ ions with two hydroxide groups bridging between Zr atoms on each side of the square and with four water molecules attached to each Zr atom.
The mineral malachite
Malachite
Malachite is a copper carbonate mineral, with the formula Cu2CO32. This green-colored mineral crystallizes in the monoclinic crystal system, and most often forms botryoidal, fibrous, or stalagmitic masses. Individual crystals are rare but do occur as slender to acicular prisms...
is a typical example of a basic carbonate. The formula, Cu2CO3(OH)2 shows that it is half-way between copper carbonate and copper hydroxide. Indeed, in the past the formula was written as CuCO3·Cu(OH)2. The crystal structure
Crystal structure
In mineralogy and crystallography, crystal structure is a unique arrangement of atoms or molecules in a crystalline liquid or solid. A crystal structure is composed of a pattern, a set of atoms arranged in a particular way, and a lattice exhibiting long-range order and symmetry...
is made up of copper, carbonate and hydroxide ions. The mineral atacamite
Atacamite
Atacamite is a copper halide mineral: a copper chloride hydroxide with formula Cu2Cl3.It was first described for deposits in the Atacama Desert of Chile in 1801....
is an example of a basic chloride. It has the formula, Cu2Cl(OH)3. In this case the composition is nearer to that of the hydroxide than that of the chloride, CuCl2·3Cu(OH)2. Copper forms hydroxy phosphate (libethenite
Libethenite
Libethenite is a rare copper phosphate hydroxide mineral. It forms striking, dark green orthorhombic crystals. It was discovered in 1823 in Ľubietová, Slovakia and is named after the German name of that locality .-References:...
), arsenate (olivenite
Olivenite
Olivenite is a copper arsenate mineral, formula Cu2AsO4OH. It crystallizes in the monoclinic system , and is sometimes found in small brilliant crystals of simple prismatic habit terminated by domal faces...
), sulfate (brochantite
Brochantite
Brochantite is a sulfate mineral, one of a number of cupric sulfates. Its chemical formula is CuSO4·3Cu2. Formed in arid climates or in rapidly oxidizing copper sulfide deposits, it is named for its discoverer, the French geologist and mineralogist, A. J. M...
) and nitrate compounds. White lead
White lead
White lead is the chemical compound 2·Pb2. It was formerly used as an ingredient for lead paint and a cosmetic called Venetian Ceruse, because its opaque quality made it a good pigment. However, it tended to cause lead poisoning, and its use has been banned in most countries.White lead has been...
is a basic lead
Lead
Lead is a main-group element in the carbon group with the symbol Pb and atomic number 82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed...
carbonate, (PbCO3)2·Pb(OH)2, which has been used as a white pigment because of its opaque quality, though its use is now restricted because it can be a source for lead poisoning
Lead poisoning
Lead poisoning is a medical condition caused by increased levels of the heavy metal lead in the body. Lead interferes with a variety of body processes and is toxic to many organs and tissues including the heart, bones, intestines, kidneys, and reproductive and nervous systems...
.
Structural chemistry
The hydroxide ion appears to rotate freely in crystals of the heavier alkali metal hydroxides at higher temperatures so as to present itself as a spherical ion, with an effective ionic radiusIonic radius
Ionic radius, rion, is the radius of an atom's ion. Although neither atoms nor ions have sharp boundaries, it is important to treat them as if they are hard spheres with radii such that the sum of ionic radii of the cation and anion gives the distance between the ions in a crystal lattice...
of about 153 pm. Thus, the high-temperature forms of KOH and NaOH have the sodium chloride structure, which gradually freezes in a monocinically distorted sodium chloride structure at temperatures below about 300 °C. The OH groups still rotate even at room temperature around their symmetry axes and, therefore, cannot be detected by X-ray diffraction. The room-temperature form of NaOH has the thallium iodide
Thallium iodide
Thallium iodide can refer to:* Thallium iodide , TlI* Thallium iodide , TlI3* Thallium iodide , TlI4...
structure. LiOH, however, has a layered structure, made up of tetrahedral Li(OH)4 and (OH)Li4 units. This is consistent with the weakly basic character of LiOH in solution, indicating that the Li-OH bond has much covalent character.
The hydroxide ion displays cylindrical symmetry in hydroxides of divalent metals Ca, Cd, Mn, Fe, and Co. For example, magnesium hydroxide, Mg(OH)2 (brucite
Brucite
Brucite is the mineral form of magnesium hydroxide, with the chemical formula Mg2. It is a common alteration product of periclase in marble; a low-temperature hydrothermal vein mineral in metamorphosed limestones and chlorite schists; and formed during serpentinization of dunites...
) crystallizes with the cadmium iodide
Cadmium iodide
Cadmium iodide, CdI2, is a chemical compound of cadmium and iodine. It is notable for its crystal structure, which is typical for compounds of the form MX2 with strong polarization effects.-Uses:...
layer structure, with a kind of close-packing of magnesium and hydroxide ions.
The amphoteric hydroxide Al(OH)3 has four major crystalline forms: gibbsite
Gibbsite
Gibbsite, Al3, is one of the mineral forms of aluminium hydroxide. It is often designated as γ-Al3 . It is also sometimes called hydrargillite ....
(most stable), bayerite, nordstrandite and doyleite.Crystal structures are illustrated at Web mineral: Gibbsite, Bayerite, Norstrandite and Doyleite
All these polymorphs are built up of double layers of hydroxide ions – the aluminium atoms on two-thirds of the octahedral holes between the two layers – and differ only in the stacking sequence of the layers. The structures are similar to the brucite structure. However, whereas the brucite structure can be described as a close-packed structure in gibbsite the OH groups on the underside of one layer rest on the groups of the layer below. This arrangement led to the suggestion that there are directional bonds between OH groups in adjacent layers. This is an unusual form of hydrogen bond
Hydrogen bond
A hydrogen bond is the attractive interaction of a hydrogen atom with an electronegative atom, such as nitrogen, oxygen or fluorine, that comes from another molecule or chemical group. The hydrogen must be covalently bonded to another electronegative atom to create the bond...
ing since the two hydroxide ion involved would be expected to point away from each other. The hydrogen atoms have been located by neutron diffraction
Neutron diffraction
Neutron diffraction or elastic neutron scattering is the application of neutron scattering to the determination of the atomic and/or magnetic structure of a material: A sample to be examined is placed in a beam of thermal or cold neutrons to obtain a diffraction pattern that provides information of...
experiments on αAlO(OH) (diaspore
Diaspore
Diaspore is a native aluminium oxide hydroxide, α-AlO, crystallizing in the orthorhombic system and isomorphous with goethite. It occurs sometimes as flattened crystals, but usually as lamellar or scaly masses, the flattened surface being a direction of perfect cleavage on which the lustre is...
). The O-H-O distance is very short, at 265 pm; the hydrogen is not equidistant between the oxygen atoms and the short OH bond makes an angle of 12° with the O-O line. A similar type of hydrogen bond has been proposed for other amphoteric hydroxides, including Be(OH)2, Zn(OH)2 and Fe(OH)3
A number of mixed hydroxides are known with stoichiometry A3MIII(OH)6, A2MIV(OH)6 and AMV(OH)6. As the formula suggests these substances contain M(OH)6 octahedral structural units. Layered double hydroxides
Layered double hydroxides
Layered double hydroxides comprise an unusual class of layered materials with positively charged layers and charge balancing anions located in the interlayer region. This is unusual in solid state chemistry: many more families of materials have negatively charged layers and cations in the...
may be represented by the formula [Mz+1–xM3+x(OH)2]q+(Xn–)q/n·yH2O. Most commonly, z = 2, and M2+ = Ca2+, Mg2+, Mn2+, Fe2+, Co2+, Ni2+, Cu2+ or Zn2+; hence q = x.
In organic reactions
Potassium hydroxidePotassium hydroxide
Potassium hydroxide is an inorganic compound with the formula KOH, commonly called caustic potash.Along with sodium hydroxide , this colorless solid is a prototypical strong base. It has many industrial and niche applications. Most applications exploit its reactivity toward acids and its corrosive...
and sodium hydroxide are two well-known reagents in organic chemistry
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
.
Base catalysis
The hydroxide ion may act as a base catalyst. The base abstracts a proton from a weak acid to give an intermediate that goes on to react with another reagent. Common substrates for proton abstraction are alcoholAlcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s, phenol
Phenol
Phenol, also known as carbolic acid, phenic acid, is an organic compound with the chemical formula C6H5OH. It is a white crystalline solid. The molecule consists of a phenyl , bonded to a hydroxyl group. It is produced on a large scale as a precursor to many materials and useful compounds...
s, amine
Amine
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines,...
s and carbon acids. The pKa
Acid dissociation constant
An acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
value for dissociation of a C-H bond is extremely high, but the pKa alpha hydrogens of a carbonyl compound are about 3 log units lower. Typical pKa values are 16.7 for acetaldehyde
Acetaldehyde
Acetaldehyde is an organic chemical compound with the formula CH3CHO or MeCHO. It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale industrially. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants as part...
and 19 for acetone
Acetone
Acetone is the organic compound with the formula 2CO, a colorless, mobile, flammable liquid, the simplest example of the ketones.Acetone is miscible with water and serves as an important solvent in its own right, typically as the solvent of choice for cleaning purposes in the laboratory...
. Dissociation can occur in the presence of a suitable base.
- RC(O)CH2R' + B RC(O)CH–R' + BH+
The base should have a pKa value not less than about 4 log units smaller or the equilibrium will lie almost completely to the left.
The hydroxide ion by itself is not a strong enough base, but it can be converted in one by adding sodium hydroxide to ethanol
- OH– + EtOH EtO– + H2O
to produce the ethoxide
Alkoxide
An alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They can be written as RO−, where R is the organic substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands...
ion. The pKa
Acid dissociation constant
An acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
for self-dissociation of ethanol
Ethanol
Ethanol, also called ethyl alcohol, pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid. It is a psychoactive drug and one of the oldest recreational drugs. Best known as the type of alcohol found in alcoholic beverages, it is also used in thermometers, as a...
is about 16 so the alkoxide ion is a strong enough base The addition of an alcohol to an aldehyde to form a hemiacetal
Hemiacetal
Hemiacetals and hemiketals are compounds that are derived from aldehydes and ketones respectively. The Greek word hèmi means half...
is an example of a reaction that can be catalyzed by the presence of hydroxide. Hydroxide can also act as a Lewis-base catalyst.
As a nucleophilic reagent
The hydroxide ion is intermediate in nucleophilicity between the fluorideFluoride
Fluoride is the anion F−, the reduced form of fluorine when as an ion and when bonded to another element. Both organofluorine compounds and inorganic fluorine containing compounds are called fluorides. Fluoride, like other halides, is a monovalent ion . Its compounds often have properties that are...
ion, F−, and the amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
ion, NH2−. The hydrolysis of an ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
,
- R1C(O)OR2 + H2O R1C(O)OH + HOR2
also known as saponification
Saponification
Saponification is a process that produces soap, usually from fats and lye. In technical terms, saponification involves base hydrolysis of triglycerides, which are esters of fatty acids, to form the sodium salt of a carboxylate. In addition to soap, such traditional saponification processes...
is an example of a nucleophilic acyl substitution
Nucleophilic acyl substitution
Nucleophilic acyl substitution describes the substitution reaction involving nucleophiles and acyl compounds. Acyl compounds are carboxylic acid derivatives including esters, amides and acid halides...
with the hydroxide ion acting as a nucleophile. In this case the leaving group is an alkoxide
Alkoxide
An alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They can be written as RO−, where R is the organic substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands...
ion, which immediately removes a proton from a water molecule to form an alcohol
Alcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
. In the manufacture of soap, sodium chloride is added to salt out the sodium salt of the carboxylic acid; this is an example of the application of the common ion effect.
Other cases where hydroxide can act as a nucleophilic reagent are amide
Amide
In chemistry, an amide is an organic compound that contains the functional group consisting of a carbonyl group linked to a nitrogen atom . The term refers both to a class of compounds and a functional group within those compounds. The term amide also refers to deprotonated form of ammonia or an...
hydrolysis, the Cannizzaro reaction
Cannizzaro reaction
The Cannizzaro reaction, named after its discoverer Stanislao Cannizzaro, is a chemical reaction that involves the base-induced disproportionation of an aldehyde lacking a hydrogen atom in the alpha position...
, nucleophilic aliphatic substitution, nucleophilic aromatic substitution
Nucleophilic aromatic substitution
right|300px|Aromatic nucleophilic substitutionA nucleophilic aromatic substitution is a substitution reaction in organic chemistry in which the nucleophile displaces a good leaving group, such as a halide, on an aromatic ring...
and in elimination reaction
Elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one or two-step mechanism...
s. The reaction medium for KOH and NaOH is usually water but with a phase-transfer catalyst the hydroxide anion can be shuttled into an organic solvent as well, for example in the generation of dichlorocarbene
Dichlorocarbene
Dichlorocarbene is a carbene commonly encountered in organic chemistry. This reactive intermediate with chemical formula CCl2 is easily available by reaction of chloroform and a base such as potassium t-butoxide or sodium hydroxide dissolved in water...
.
Hydroxyl groups in organic compounds
Organic compounds such as alcoholAlcohol
In chemistry, an alcohol is an organic compound in which the hydroxy functional group is bound to a carbon atom. In particular, this carbon center should be saturated, having single bonds to three other atoms....
s, phenols
Phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of a hydroxyl group bonded directly to an aromatic hydrocarbon group...
and carboxylic acid
Carboxylic acid
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group...
s contain hydroxyl groups. Each class of compound undergoes reactions specific to that class.

