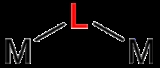
Bridging ligand
Encyclopedia
A bridging ligand is a ligand
that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually restricted to small ligands such as pseudohalides or to ligands that are specifically designed to link two metals.
In naming a complex wherein a single atom bridges two metals, the bridging ligand is preceded by "μ" with a superscript number denoting the number of metals bound to the bridging ligand is bound. μ2 is often denoted simply as μ.
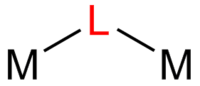
Cyanide usually bridges via M-NC-M' linkages, unlike the other entries on this list.
Many organic ligands form strong bridges between metal centers. Many common examples include derivatives of the above inorganic ligands (R = alkyl, aryl):
and the related Carboxylate, PO43−
, and the polyoxometallates. Several organophosphorus ligands have been developed that bridge pairs of metals, a well-known example being Ph2PCH2PPh2.
Ligand
In coordination chemistry, a ligand is an ion or molecule that binds to a central metal atom to form a coordination complex. The bonding between metal and ligand generally involves formal donation of one or more of the ligand's electron pairs. The nature of metal-ligand bonding can range from...
that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually restricted to small ligands such as pseudohalides or to ligands that are specifically designed to link two metals.
In naming a complex wherein a single atom bridges two metals, the bridging ligand is preceded by "μ" with a superscript number denoting the number of metals bound to the bridging ligand is bound. μ2 is often denoted simply as μ.

Illustrative bridging ligands
Virtually all ligands are known to bridge, with the exception of amines and ammonia. Particularly common inorganic bridging ligands include- OH−HydroxideHydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
, - O2−OxideAn oxide is a chemical compound that contains at least one oxygen atom in its chemical formula. Metal oxides typically contain an anion of oxygen in the oxidation state of −2....
, - S2−SulfideA sulfide is an anion of sulfur in its lowest oxidation state of 2-. Sulfide is also a slightly archaic term for thioethers, a common type of organosulfur compound that are well known for their bad odors.- Properties :...
, - SH−BisulfideThe bisulfide ion, also called hydrogensulfide or hydrosulfide, is the anion with the formula [HS]− . This species is the conjugate base of hydrogen sulfide and in its turn also dissociates in sulfide:A variety of salts are known, including sodium hydrosulfide and potassium hydrosulfide...
, - NH2−
- NH2− (imido)
- N3− (nitrido)
- COCarbon monoxideCarbon monoxide , also called carbonous oxide, is a colorless, odorless, and tasteless gas that is slightly lighter than air. It is highly toxic to humans and animals in higher quantities, although it is also produced in normal animal metabolism in low quantities, and is thought to have some normal...
- HalideHalideA halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
s - HydrideHydrideIn chemistry, a hydride is the anion of hydrogen, H−, or, more commonly, a compound in which one or more hydrogen centres have nucleophilic, reducing, or basic properties. In compounds that are regarded as hydrides, hydrogen is bonded to a more electropositive element or group...
- CyanideCyanideA cyanide is a chemical compound that contains the cyano group, -C≡N, which consists of a carbon atom triple-bonded to a nitrogen atom. Cyanides most commonly refer to salts of the anion CN−. Most cyanides are highly toxic....
Cyanide usually bridges via M-NC-M' linkages, unlike the other entries on this list.
Many organic ligands form strong bridges between metal centers. Many common examples include derivatives of the above inorganic ligands (R = alkyl, aryl):
- OR−AlkoxideAn alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They can be written as RO−, where R is the organic substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands...
, - SR−,
- NR2−
- NR2− (imido)
- P3− (phosphido)
- PR2− (phosphido, note the ambiguity with the preceding entry)
- PR2− (phosphinidino)
Polyfunctional ligands
Polyfunctional ligands can attach to metals in many ways and thus can bridge metals in diverse ways, including sharing of one atom or using several atoms. Examples of such polyatomic ligands are the oxoanions CO32−Carbonate
In chemistry, a carbonate is a salt of carbonic acid, characterized by the presence of the carbonate ion, . The name may also mean an ester of carbonic acid, an organic compound containing the carbonate group C2....
and the related Carboxylate, PO43−
Phosphate
A phosphate, an inorganic chemical, is a salt of phosphoric acid. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry and biogeochemistry or ecology. Inorganic phosphates are mined to obtain phosphorus for use in...
, and the polyoxometallates. Several organophosphorus ligands have been developed that bridge pairs of metals, a well-known example being Ph2PCH2PPh2.
Examples
| Compound | Formula | Description |
|---|---|---|
.png) |
{(Fe(III)(OH2)4)2(µ-OH)2}4+ | In this example hydroxide Hydroxide Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a... plays the role of a μ2 bridging ligand. Notice in the name of the compound μ2 has been simplified to μ. |
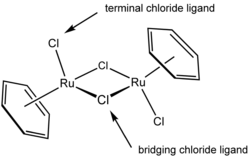 |
(η6-C6H6)2Ru2Cl2(μ-Cl)2 | In this particular complex, two chloride Chloride The chloride ion is formed when the element chlorine, a halogen, picks up one electron to form an anion Cl−. The salts of hydrochloric acid HCl contain chloride ions and can also be called chlorides. The chloride ion, and its salts such as sodium chloride, are very soluble in water... ligands are terminal and two are μ2 bridging. The η in the beginning of the formula denotes the hapticity Hapticity The term hapticity is used to describe how a group of contiguous atoms of a ligand are coordinated to a central atom. Hapticity of a ligand is indicated by the Greek character 'eta', η. A superscripted number following the η denotes the number of contiguous atoms of the ligand that are bound to... of the benzene ligands. |
 |
B2H6 | This classic borane compound, diborane features two μ2 bridging hydrides. |
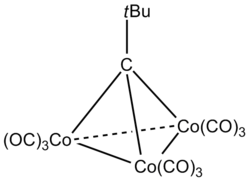 |
(Co(CO)3)3(μ3-(C-tBu)) | This compound contains a μ3 bridging carbyne ligand (C-tBu). |

