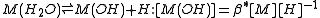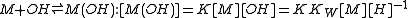
Equilibrium constant
Encyclopedia
For a general chemical equilibrium
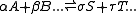
the equilibrium constant can be defined by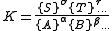
where {A} is the activity
of the chemical species A, etc. (activity is a dimensionless quantity). It is conventional to put the activities of the products in the numerator and those of the reactants in the denominator. A derivation of this expression is given below.
For equilibria in solution activity is the product of concentration
and activity coefficient
. It is common practice to determine equilibrium constants in a medium of high ionic strength
. In those circumstances the quotient of activity coefficients is effectively constant and the equilibrium constant is taken to be a concentration quotient.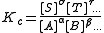
However, the value of Kc will depend on the ionic strength.
All equilibrium constants depend on temperature and pressure (or volume).
A knowledge of equilibrium constants is essential for the understanding of many natural processes such as oxygen transport by haemoglobin in blood and acid-base homeostasis
in the human body.
Stability constants, formation constants, binding constants, association constants and dissociation constants are all types of equilibrium constant. See also Determination of equilibrium constants
for experimental and computational methods.
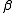 , is the constant for the formation of a complex from reagents. For example, the cumulative constant for the formation of ML2 is given by
, is the constant for the formation of a complex from reagents. For example, the cumulative constant for the formation of ML2 is given by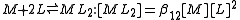
The stepwise constant, K, for the formation of the same complex from ML and L is given by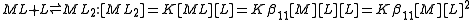
It follows that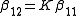
A cumulative constant can always be expressed as the product of stepwise constants. There is no agreed notation for stepwise constants, though a symbol such as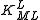 is sometimes found in the literature. It is best always to define each stability constant by reference to an equilibrium expression.
is sometimes found in the literature. It is best always to define each stability constant by reference to an equilibrium expression.
complexes of many metals are outside the range for the potentiometric method. The stability constants for those complexes were determined by competition with a weaker ligand.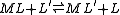
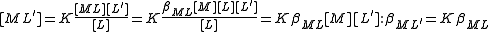
The formation constant of [Pd(CN)4]2-
was determined by the competition method.
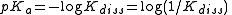
where Kdiss is a stepwise acid dissociation constant
. For bases, the base association constant, pKb is used. For any given acid or base the two constants are related by pKa + pKb = pKw, so pKa can always be used in calculations.
On the other hand stability constants for metal complexes, and binding constants for host-guest
complexes are generally expressed as association constants. When considering equilibria such as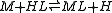
it is customary to use association constants for both ML and HL. Also, in generalized computer programs dealing with equilibrium constants it is general practice to use cumulative constants rather than stepwise constants and to omit ionic charges from equilibrium expressions. For example, if NTA, nitrilotriacetic acid
, N(CH2CO2H)3 is designated as H3L and forms complexes ML and MHL with a metal ion M, the following expressions would apply for the dissociation constants.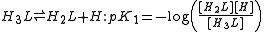
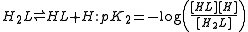
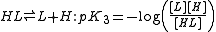
The cumulative association constants can be expressed as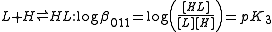
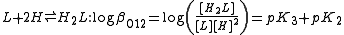
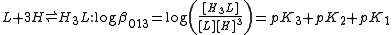
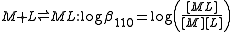
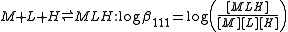
Note how the subscripts define the stoichiometry of the equilibrium product.
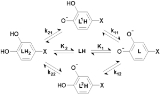
has two non-equivalent hydroxyl groups which may be deprotonated. Denoting L-Dopa as LH2, the following diagram shows all the species that may be formed (X=CH2CH(NH2)CO2H)
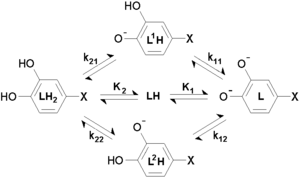
The first protonation constants are
The concentration of LH- is the sum of the concentrations of the two micro-species. Therefore, the equilibrium constant for the reaction, the macro-constant, is the sum of the micro-constants.
In the same way,
Lastly, the cumulative constant is
Thus, although there are six micro-and macro-constants, only three of them are mutually independent. Moreover, the isomerization constant, Ki, is equal to the ratio of the microconstants.
In L-Dopa the isomerization constant is 0.9, so the micro-species L1H and L2H have almost equal concentrations at all pH values.
In general a macro-constant is equal to the sum of all the micro-constants and the occupancy of each site is proportional to the micro-constant. The site of protonation can be very important, for example, for biological activity.
Micro-constants cannot be determined individually by the usual methods
, which give macro-constants. Methods which have been used to determine micro-constants include:
is defined in terms of the activity
of the hydrogen ion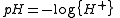
pH is measured by means of a glass electrode, a mixed equilibrium constant, also known as a Brønsted constant, may result.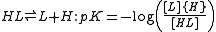
It all depends on whether the electrode is calibrated by reference to solutions of known activity or known concentration. In the latter case the equilibrium constant would be a concentration quotient. If the electrode is calibrated in terms of known hydrogen ion concentrations it would be better to write p[H] rather than pH, but this suggestion is not generally adopted.
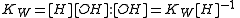
The first step in metal ion hydrolysis
can be expressed in two different ways
It follows that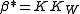 . Hydrolysis constants are usually reported in the
. Hydrolysis constants are usually reported in the 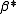 form and this leads to them appearing to have strange values. For example, if lgK=4 and lg KW=-14, lg
form and this leads to them appearing to have strange values. For example, if lgK=4 and lg KW=-14, lg 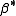 = 4 -14 = -10. In general when the hydrolysis product contains n hydroxide groups lg
= 4 -14 = -10. In general when the hydrolysis product contains n hydroxide groups lg 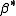 = lg K + n lg KW
= lg K + n lg KW
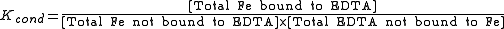
This conditional constant will vary with pH. It has a maximum at a certain pH. That is the pH where the ligand sequesters the metal most effectively.
In biochemistry equilibrium constants are often measured at a pH fixed by means of a buffer solution
. Such constants are, by definition, conditional and different values may be obtained when using different buffers.
, f, is used in place of activity. However, fugacity has the dimension
of pressure
, so it must be divided by a standard pressure, usually 1 bar, in order to produce a dimensionless quantity, f/pstd. An equilibrium constant is expressed in terms of the dimensionless quantity. For example, for the equilibrium
Fugacity is related to partial pressure
, p, by a dimensionless fugacity coefficient,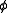 .
.
Thus, for the example,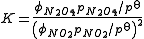
Usually the standard pressure is omitted from such expressions. Expressions for equilibrium constants in the gas phase then resemble the expression for solution equilibria with fugacity coefficient in place of activity coefficient and partial pressure in place of concentration.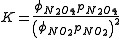
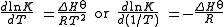
shows that when the reaction is exothermic (ΔH is negative), then K decreases with increasing temperature, in accordance with Le Chatelier's principle
is negative), then K decreases with increasing temperature, in accordance with Le Chatelier's principle
. It permits calculation of the reaction equilibrium constant at temperature T2 if the reaction constant at T1 is known and the standard reaction enthalpy can be assumed to be independent of temperature even though each standard enthalpy change is defined at a different temperature. However, this assumption is valid only for small temperature differences T2 - T1. In fact standard thermodynamic arguments can be used to show that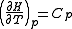
where Cp is the heat capacity at constant pressure.
The equilibrium constant is related to the standard Gibbs energy change of reaction as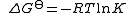
where ΔG is the standard Gibbs energy change of reaction, R is the gas constant
is the standard Gibbs energy change of reaction, R is the gas constant
, and T the absolute temperature.
If the equilibrium constant has been determined and the standard reaction enthalpy has also been determined, by calorimetry, for example, this equation allows the standard entropy change for the reaction to be derived.
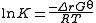
where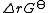 is the reaction standard Gibbs energy, which is the sum of the standard Gibbs energies of the reaction products minus the sum of standard Gibbs energies of reactants.
is the reaction standard Gibbs energy, which is the sum of the standard Gibbs energies of the reaction products minus the sum of standard Gibbs energies of reactants.
Here, the term "standard" denotes the ideal behaviour (i.e., an infinite dilution) and a hypothetical standard concentration (typically 1 mol/kg). It does not imply any particular temperature or pressure because, although contrary to IUPAC recommendation, it is more convenient when describing aqueous systems over a wide temperature and pressure ranges.
The standard Gibbs energy (for each species or for the entire reaction) can be represented (from the basic definitions) as:
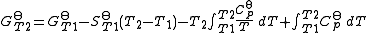
In the above equation, the effect of temperature on Gibbs energy (and thus on the equilibrium constant) is ascribed entirely to heat capacity. To evaluate the integrals in this equation, the form of the dependence of heat capacity on temperature needs to be known.
Now, if one expresses the standard heat capacity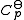 , as a function of absolute temperature using correlations of the following forms:
, as a function of absolute temperature using correlations of the following forms:
then the integrals can be evaluated and the following final form is obtained:
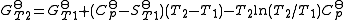
The constants A,B,C,a,b and the absolute entropy,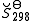 , required for evaluation of
, required for evaluation of 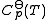 , as well as the values of G298 K and S298 K for many species are tabularized in the literature.
, as well as the values of G298 K and S298 K for many species are tabularized in the literature.
reactant/products (i.e., when reactants and products are solids or liquid) as well as gaseous ones.
For a gaseous-reaction example, one may consider the well-studied reaction of hydrogen with nitrogen to produce ammonia:
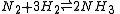
If the pressure is increased by an addition of an inert gas, then neither the composition at equilibrium nor the equilibrium constant are appreciably affected (because the partial pressures remain constant, assuming an ideal-gas behaviour of all gases involved). However, the composition at equilibrium will depend appreciably on pressure when:
In the example reaction above, the number of moles changes from 4 to 2, and an increase of pressure by system compression will result in appreciably more ammonia in the equilibrium mixture. In the general case of a gaseous reaction:
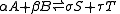
the change of mixture composition with pressure can be quantified using:
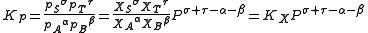
where p denote the partial pressures of the components, P is the total system pressure, X denote the mole fraction, Kp is the equilibrium constant expressed in terms of partial pressures and KX is the equilibrium constant expressed in terms of mol fractions.
The above change in composition is in accordance with Le Chatelier's principle
and does not involve any change of the equilibrium constant with the total system pressure. Indeed, for ideal-gas reactions Kp is independent of pressure.
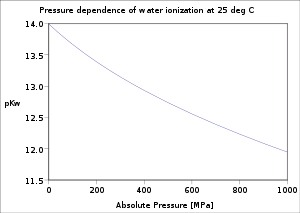 In a condensed phase, the pressure dependence of the equilibrium constant is associated with the reaction molar volume. For reaction:
In a condensed phase, the pressure dependence of the equilibrium constant is associated with the reaction molar volume. For reaction:
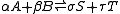
the reaction molar volume is:

where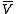 denotes a partial molar volume of a reactant or a product.
denotes a partial molar volume of a reactant or a product.
For the above reaction, one can expect the change of the reaction equilibrium constant (based either on mole-fraction or molal-concentration scale) with pressure at constant temperature to be:
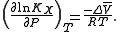
The matter is complicated as partial molar volume is itself dependent on pressure.
of the system, along with the Law of Mass Action. μ is the chemical potential, N is the number of molecules in the system, S is the entropy of the system, T is the temperature, V is the volume and P is the pressure.
For any chemical reaction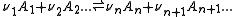
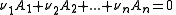
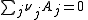
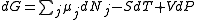
Assuming constant pressure and temperature, dT = dP = 0
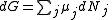
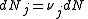
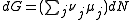
At equilibrium conditions, dG =0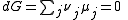
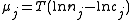
Where nj is the concentration of the species and cj is a constant dependent on the temperature but not on the concentration.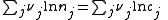
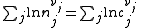
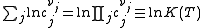
K(T) is the equilibrium constant.
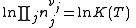
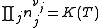
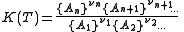
NIST Standard Reference Database 46 Critically Selected Stability Constants of Metal Complexes
Inorganic and organic acids and bases pKa data in water and DMSO
Chemical equilibrium
In a chemical reaction, chemical equilibrium is the state in which the concentrations of the reactants and products have not yet changed with time. It occurs only in reversible reactions, and not in irreversible reactions. Usually, this state results when the forward reaction proceeds at the same...
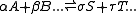
the equilibrium constant can be defined by
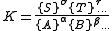
where {A} is the activity
Activity (chemistry)
In chemical thermodynamics, activity is a measure of the “effective concentration” of a species in a mixture, meaning that the species' chemical potential depends on the activity of a real solution in the same way that it would depend on concentration for an ideal solution.By convention, activity...
of the chemical species A, etc. (activity is a dimensionless quantity). It is conventional to put the activities of the products in the numerator and those of the reactants in the denominator. A derivation of this expression is given below.
For equilibria in solution activity is the product of concentration
Concentration
In chemistry, concentration is defined as the abundance of a constituent divided by the total volume of a mixture. Four types can be distinguished: mass concentration, molar concentration, number concentration, and volume concentration...
and activity coefficient
Activity coefficient
An activity coefficient is a factor used in thermodynamics to account for deviations from ideal behaviour in a mixture of chemical substances. In an ideal mixture, the interactions between each pair of chemical species are the same and, as a result, properties of the mixtures can be expressed...
. It is common practice to determine equilibrium constants in a medium of high ionic strength
Ionic strength
The ionic strength of a solution is a measure of the concentration of ions in that solution. Ionic compounds, when dissolved in water, dissociate into ions. The total electrolyte concentration in solution will affect important properties such as the dissociation or the solubility of different salts...
. In those circumstances the quotient of activity coefficients is effectively constant and the equilibrium constant is taken to be a concentration quotient.
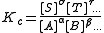
However, the value of Kc will depend on the ionic strength.
All equilibrium constants depend on temperature and pressure (or volume).
A knowledge of equilibrium constants is essential for the understanding of many natural processes such as oxygen transport by haemoglobin in blood and acid-base homeostasis
Acid-base homeostasis
Acid–base homeostasis is the part of human homeostasis concerning the proper balance between acids and bases, in other words, the pH. The body is very sensitive to its pH level, so strong mechanisms exist to maintain it...
in the human body.
Stability constants, formation constants, binding constants, association constants and dissociation constants are all types of equilibrium constant. See also Determination of equilibrium constants
Determination of equilibrium constants
Equilibrium constants are determined in order to quantify chemical equilibria. When an equilibrium constant is expressed as a concentration quotient,K=\frac...
for experimental and computational methods.
Cumulative and stepwise formation constants
A cumulative or overall constant, given the symbol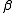 , is the constant for the formation of a complex from reagents. For example, the cumulative constant for the formation of ML2 is given by
, is the constant for the formation of a complex from reagents. For example, the cumulative constant for the formation of ML2 is given by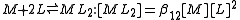
The stepwise constant, K, for the formation of the same complex from ML and L is given by
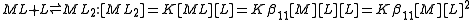
It follows that
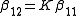
A cumulative constant can always be expressed as the product of stepwise constants. There is no agreed notation for stepwise constants, though a symbol such as
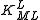 is sometimes found in the literature. It is best always to define each stability constant by reference to an equilibrium expression.
is sometimes found in the literature. It is best always to define each stability constant by reference to an equilibrium expression.Competition method
A particular use of a stepwise constant is in the determination of stability constant values outside the normal range for a given method. For example, EDTAEDTA
Ethylenediaminetetraacetic acid, widely abbreviated as EDTA , is a polyamino carboxylic acid and a colourless, water-soluble solid. Its conjugate base is named ethylenediaminetetraacetate. It is widely used to dissolve limescale. Its usefulness arises because of its role as a hexadentate ligand...
complexes of many metals are outside the range for the potentiometric method. The stability constants for those complexes were determined by competition with a weaker ligand.
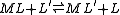
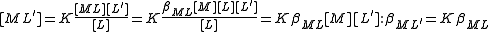
The formation constant of [Pd(CN)4]2-
Palladium(II) cyanide
Palladium cyanides are chemical species with the empirical formula Pdn-. The dicyanide is a coordination polymer which was the first pure palladium compound isolated. In his attempts to produce pure platinum metal in 1804, W.H...
was determined by the competition method.
Association and dissociation constants
In organic chemistry and biochemistry it is customary to use pKa values for acid dissociation equilibria.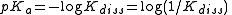
where Kdiss is a stepwise acid dissociation constant
Acid dissociation constant
An acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
. For bases, the base association constant, pKb is used. For any given acid or base the two constants are related by pKa + pKb = pKw, so pKa can always be used in calculations.
On the other hand stability constants for metal complexes, and binding constants for host-guest
Host-guest chemistry
In supramolecular chemistry, host-guest chemistry describes complexes that are composed of two or more molecules or ions that are held together in unique structural relationships by forces other than those of full covalent bonds. Host-guest chemistry encompasses the idea of molecular recognition...
complexes are generally expressed as association constants. When considering equilibria such as
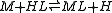
it is customary to use association constants for both ML and HL. Also, in generalized computer programs dealing with equilibrium constants it is general practice to use cumulative constants rather than stepwise constants and to omit ionic charges from equilibrium expressions. For example, if NTA, nitrilotriacetic acid
Nitrilotriacetic acid
Nitrilotriacetic acid , C6H9NO6, is a polyamino carboxylic acid and is used as a chelating agent which forms coordination compounds with metal ions such as Ca2+, Cu2+ or Fe3+.The uses of NTA are similar to that of EDTA...
, N(CH2CO2H)3 is designated as H3L and forms complexes ML and MHL with a metal ion M, the following expressions would apply for the dissociation constants.
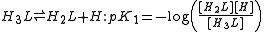
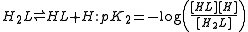
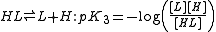
The cumulative association constants can be expressed as
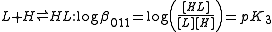
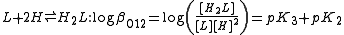
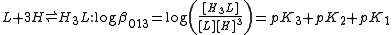
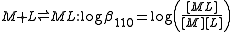
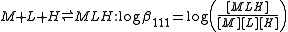
Note how the subscripts define the stoichiometry of the equilibrium product.
Micro-constants
When two or more sites in an asymmetrical molecule may be involved in an equilibrium reaction there are more than one possible equilibrium constants. For example, the molecule L-dopaLevodopa
L-DOPA is a chemical that is made and used as part of the normal biology of some animals and plants. Some animals including humans make it via biosynthesis from the amino acid L-tyrosine. L-DOPA is the precursor to the neurotransmitters dopamine, norepinephrine , and epinephrine collectively...
has two non-equivalent hydroxyl groups which may be deprotonated. Denoting L-Dopa as LH2, the following diagram shows all the species that may be formed (X=CH2CH(NH2)CO2H)

The first protonation constants are
- [L1H] = k11[L][H], [L2H] = k12[L][H]
The concentration of LH- is the sum of the concentrations of the two micro-species. Therefore, the equilibrium constant for the reaction, the macro-constant, is the sum of the micro-constants.
- K1 = k11 + k12
In the same way,
- K2 = k21 + k22
Lastly, the cumulative constant is
- β2=K1K2=k11k21=k12k22
Thus, although there are six micro-and macro-constants, only three of them are mutually independent. Moreover, the isomerization constant, Ki, is equal to the ratio of the microconstants.
- Ki=k11/k12
In L-Dopa the isomerization constant is 0.9, so the micro-species L1H and L2H have almost equal concentrations at all pH values.
In general a macro-constant is equal to the sum of all the micro-constants and the occupancy of each site is proportional to the micro-constant. The site of protonation can be very important, for example, for biological activity.
Micro-constants cannot be determined individually by the usual methods
Determination of equilibrium constants
Equilibrium constants are determined in order to quantify chemical equilibria. When an equilibrium constant is expressed as a concentration quotient,K=\frac...
, which give macro-constants. Methods which have been used to determine micro-constants include:
- blocking one of the sites, for example by methylation of a hydroxyl group, to determine one of the micro-constants
- using a spectroscopic technique, such as infrared spectroscopyInfrared spectroscopyInfrared spectroscopy is the spectroscopy that deals with the infrared region of the electromagnetic spectrum, that is light with a longer wavelength and lower frequency than visible light. It covers a range of techniques, mostly based on absorption spectroscopy. As with all spectroscopic...
, where the different micro-species give different signals. - applying mathematical procedures to 13C NMR data.
pH considerations (Brønsted constants)
pHPH
In chemistry, pH is a measure of the acidity or basicity of an aqueous solution. Pure water is said to be neutral, with a pH close to 7.0 at . Solutions with a pH less than 7 are said to be acidic and solutions with a pH greater than 7 are basic or alkaline...
is defined in terms of the activity
Activity (chemistry)
In chemical thermodynamics, activity is a measure of the “effective concentration” of a species in a mixture, meaning that the species' chemical potential depends on the activity of a real solution in the same way that it would depend on concentration for an ideal solution.By convention, activity...
of the hydrogen ion
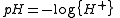
pH is measured by means of a glass electrode, a mixed equilibrium constant, also known as a Brønsted constant, may result.
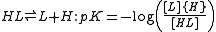
It all depends on whether the electrode is calibrated by reference to solutions of known activity or known concentration. In the latter case the equilibrium constant would be a concentration quotient. If the electrode is calibrated in terms of known hydrogen ion concentrations it would be better to write p[H] rather than pH, but this suggestion is not generally adopted.
Hydrolysis constants
In aqueous solution the concentration of the hydroxide ion is related to the concentration of the hydrogen ion by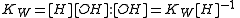
The first step in metal ion hydrolysis
Hydrolysis
Hydrolysis is a chemical reaction during which molecules of water are split into hydrogen cations and hydroxide anions in the process of a chemical mechanism. It is the type of reaction that is used to break down certain polymers, especially those made by condensation polymerization...
can be expressed in two different ways
It follows that
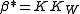 . Hydrolysis constants are usually reported in the
. Hydrolysis constants are usually reported in the 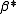 form and this leads to them appearing to have strange values. For example, if lgK=4 and lg KW=-14, lg
form and this leads to them appearing to have strange values. For example, if lgK=4 and lg KW=-14, lg 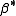 = 4 -14 = -10. In general when the hydrolysis product contains n hydroxide groups lg
= 4 -14 = -10. In general when the hydrolysis product contains n hydroxide groups lg 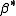 = lg K + n lg KW
= lg K + n lg KWConditional constants
Conditional constants, also known as apparent constants, are concentration quotients which are not true equilibrium constants but can be derived from them. A very common instance is where pH is fixed at a particular value. For example, in the case of iron(III) interacting with EDTA, a conditional constant could be defined by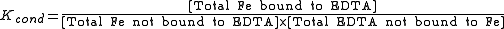
This conditional constant will vary with pH. It has a maximum at a certain pH. That is the pH where the ligand sequesters the metal most effectively.
In biochemistry equilibrium constants are often measured at a pH fixed by means of a buffer solution
Buffer solution
A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. It has the property that the pH of the solution changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a...
. Such constants are, by definition, conditional and different values may be obtained when using different buffers.
Gas phase equilibria
For equilibria in a gas phase, fugacityFugacity
In chemical thermodynamics, the fugacity of a real gas is an effective pressure which replaces the true mechanical pressure in accurate chemical equilibrium calculations. It is equal to the pressure of an ideal gas which has the same chemical potential as the real gas. For example, nitrogen gas ...
, f, is used in place of activity. However, fugacity has the dimension
Dimension
In physics and mathematics, the dimension of a space or object is informally defined as the minimum number of coordinates needed to specify any point within it. Thus a line has a dimension of one because only one coordinate is needed to specify a point on it...
of pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
, so it must be divided by a standard pressure, usually 1 bar, in order to produce a dimensionless quantity, f/pstd. An equilibrium constant is expressed in terms of the dimensionless quantity. For example, for the equilibrium
- 2NO2 N2O4
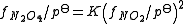
Fugacity is related to partial pressure
Partial pressure
In a mixture of ideal gases, each gas has a partial pressure which is the pressure which the gas would have if it alone occupied the volume. The total pressure of a gas mixture is the sum of the partial pressures of each individual gas in the mixture....
, p, by a dimensionless fugacity coefficient,
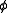 .
.
- f =
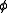 p
p
Thus, for the example,
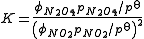
Usually the standard pressure is omitted from such expressions. Expressions for equilibrium constants in the gas phase then resemble the expression for solution equilibria with fugacity coefficient in place of activity coefficient and partial pressure in place of concentration.
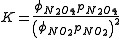
Temperature dependence
The van 't Hoff equation.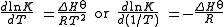
shows that when the reaction is exothermic (ΔH
 is negative), then K decreases with increasing temperature, in accordance with Le Chatelier's principle
is negative), then K decreases with increasing temperature, in accordance with Le Chatelier's principleLe Châtelier's principle
In chemistry, Le Chatelier's principle, also called the Chatelier's principle, can be used to predict the effect of a change in conditions on a chemical equilibrium. The principle is named after Henry Louis Le Chatelier and sometimes Karl Ferdinand Braun who discovered it independently...
. It permits calculation of the reaction equilibrium constant at temperature T2 if the reaction constant at T1 is known and the standard reaction enthalpy can be assumed to be independent of temperature even though each standard enthalpy change is defined at a different temperature. However, this assumption is valid only for small temperature differences T2 - T1. In fact standard thermodynamic arguments can be used to show that
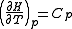
where Cp is the heat capacity at constant pressure.
The equilibrium constant is related to the standard Gibbs energy change of reaction as
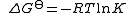
where ΔG
 is the standard Gibbs energy change of reaction, R is the gas constant
is the standard Gibbs energy change of reaction, R is the gas constantGas constant
The gas constant is a physical constant which is featured in many fundamental equations in the physical sciences, such as the ideal gas law and the Nernst equation. It is equivalent to the Boltzmann constant, but expressed in units of energy The gas constant (also known as the molar, universal,...
, and T the absolute temperature.
If the equilibrium constant has been determined and the standard reaction enthalpy has also been determined, by calorimetry, for example, this equation allows the standard entropy change for the reaction to be derived.
A more complex formulation
The calculation of KT2 from known KT1 can be approached as follows if standard thermodynamic properties are available. The effect of temperature on equilibrium constant is equivalent to the effect of temperature on Gibbs energy because: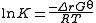
where
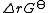 is the reaction standard Gibbs energy, which is the sum of the standard Gibbs energies of the reaction products minus the sum of standard Gibbs energies of reactants.
is the reaction standard Gibbs energy, which is the sum of the standard Gibbs energies of the reaction products minus the sum of standard Gibbs energies of reactants.Here, the term "standard" denotes the ideal behaviour (i.e., an infinite dilution) and a hypothetical standard concentration (typically 1 mol/kg). It does not imply any particular temperature or pressure because, although contrary to IUPAC recommendation, it is more convenient when describing aqueous systems over a wide temperature and pressure ranges.
The standard Gibbs energy (for each species or for the entire reaction) can be represented (from the basic definitions) as:
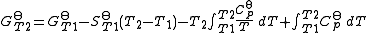
In the above equation, the effect of temperature on Gibbs energy (and thus on the equilibrium constant) is ascribed entirely to heat capacity. To evaluate the integrals in this equation, the form of the dependence of heat capacity on temperature needs to be known.
Now, if one expresses the standard heat capacity
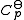 , as a function of absolute temperature using correlations of the following forms:
, as a function of absolute temperature using correlations of the following forms:
- For pure substances (solids, gas, liquid):
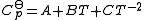
- For ionic species at T < 200 deg C:
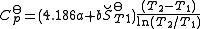
then the integrals can be evaluated and the following final form is obtained:
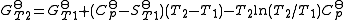
The constants A,B,C,a,b and the absolute entropy,
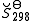 , required for evaluation of
, required for evaluation of 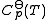 , as well as the values of G298 K and S298 K for many species are tabularized in the literature.
, as well as the values of G298 K and S298 K for many species are tabularized in the literature.Pressure dependence
The pressure dependence of the equilibrium constant is usually weak in the range of pressures normally encountered in industry, and therefore, it is usually neglected in practice. This is true for condensedCondensed matter physics
Condensed matter physics deals with the physical properties of condensed phases of matter. These properties appear when a number of atoms at the supramolecular and macromolecular scale interact strongly and adhere to each other or are otherwise highly concentrated in a system. The most familiar...
reactant/products (i.e., when reactants and products are solids or liquid) as well as gaseous ones.
For a gaseous-reaction example, one may consider the well-studied reaction of hydrogen with nitrogen to produce ammonia:
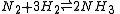
If the pressure is increased by an addition of an inert gas, then neither the composition at equilibrium nor the equilibrium constant are appreciably affected (because the partial pressures remain constant, assuming an ideal-gas behaviour of all gases involved). However, the composition at equilibrium will depend appreciably on pressure when:
- the pressure is changed by compression of the gaseous reacting system, and
- the reaction results in the change of the number of moles of gas in the system.
In the example reaction above, the number of moles changes from 4 to 2, and an increase of pressure by system compression will result in appreciably more ammonia in the equilibrium mixture. In the general case of a gaseous reaction:
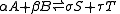
the change of mixture composition with pressure can be quantified using:
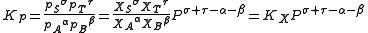
where p denote the partial pressures of the components, P is the total system pressure, X denote the mole fraction, Kp is the equilibrium constant expressed in terms of partial pressures and KX is the equilibrium constant expressed in terms of mol fractions.
The above change in composition is in accordance with Le Chatelier's principle
Le Châtelier's principle
In chemistry, Le Chatelier's principle, also called the Chatelier's principle, can be used to predict the effect of a change in conditions on a chemical equilibrium. The principle is named after Henry Louis Le Chatelier and sometimes Karl Ferdinand Braun who discovered it independently...
and does not involve any change of the equilibrium constant with the total system pressure. Indeed, for ideal-gas reactions Kp is independent of pressure.

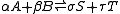
the reaction molar volume is:

where
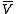 denotes a partial molar volume of a reactant or a product.
denotes a partial molar volume of a reactant or a product.For the above reaction, one can expect the change of the reaction equilibrium constant (based either on mole-fraction or molal-concentration scale) with pressure at constant temperature to be:
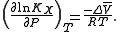
The matter is complicated as partial molar volume is itself dependent on pressure.
Derivation from Gibbs Free Energy
The mathematical definition of the equilibrium constant can be derived directly from the Gibbs Free EnergyGibbs free energy
In thermodynamics, the Gibbs free energy is a thermodynamic potential that measures the "useful" or process-initiating work obtainable from a thermodynamic system at a constant temperature and pressure...
of the system, along with the Law of Mass Action. μ is the chemical potential, N is the number of molecules in the system, S is the entropy of the system, T is the temperature, V is the volume and P is the pressure.
For any chemical reaction
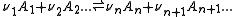
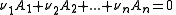
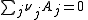
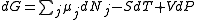
Assuming constant pressure and temperature, dT = dP = 0
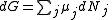
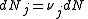
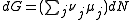
At equilibrium conditions, dG =0
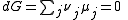
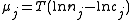
Where nj is the concentration of the species and cj is a constant dependent on the temperature but not on the concentration.
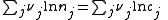
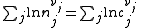
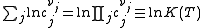
K(T) is the equilibrium constant.
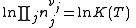
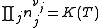
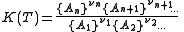
Data sources
IUPAC SC-Database A comprehensive database of published data on equilibrium constants of metal complexes and ligandsNIST Standard Reference Database 46 Critically Selected Stability Constants of Metal Complexes
Inorganic and organic acids and bases pKa data in water and DMSO
Dimethyl sulfoxide
Dimethyl sulfoxide is an organosulfur compound with the formula 2SO. This colorless liquid is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water...
External links
- Science Aid: Equilibrium Constants Explanation of Kc and Kp for High School level