
Gas constant
Encyclopedia
| Values of R |
Units (V P T −1 n−1) Ideal gas law The ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. It was first stated by Émile Clapeyron in 1834 as a combination of Boyle's law and Charles's law... |
|---|---|
| J Joule The joule ; symbol J) is a derived unit of energy or work in the International System of Units. It is equal to the energy expended in applying a force of one newton through a distance of one metre , or in passing an electric current of one ampere through a resistance of one ohm for one second... K Kelvin The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all... −1 mol Mole (unit) The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value... −1 |
|
| eV K Kelvin The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all... −1 mol Mole (unit) The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value... −1 |
|
| L Litér - External links :*... atm Atmosphere (unit) The standard atmosphere is an international reference pressure defined as 101325 Pa and formerly used as unit of pressure. For practical purposes it has been replaced by the bar which is 105 Pa... K−1 mol−1 |
|
| cal Calorie The calorie is a pre-SI metric unit of energy. It was first defined by Nicolas Clément in 1824 as a unit of heat, entering French and English dictionaries between 1841 and 1867. In most fields its use is archaic, having been replaced by the SI unit of energy, the joule... K−1 mol−1 |
|
| erg Erg An erg is the unit of energy and mechanical work in the centimetre-gram-second system of units, symbol "erg". Its name is derived from the Greek ergon, meaning "work".... K−1 mol−1 |
|
| L kPa Pascal (unit) The pascal is the SI derived unit of pressure, internal pressure, stress, Young's modulus and tensile strength, named after the French mathematician, physicist, inventor, writer, and philosopher Blaise Pascal. It is a measure of force per unit area, defined as one newton per square metre... K−1 mol−1 |
|
| m3 Cubic metre The cubic metre is the SI derived unit of volume. It is the volume of a cube with edges one metre in length. An alternative name, which allowed a different usage with metric prefixes, was the stère... Pa Pascal (unit) The pascal is the SI derived unit of pressure, internal pressure, stress, Young's modulus and tensile strength, named after the French mathematician, physicist, inventor, writer, and philosopher Blaise Pascal. It is a measure of force per unit area, defined as one newton per square metre... K−1 mol−1 |
|
| cm3 MPa K−1 mol−1 | |
| m3 bar Bar (unit) The bar is a unit of pressure equal to 100 kilopascals, and roughly equal to the atmospheric pressure on Earth at sea level. Other units derived from the bar are the megabar , kilobar , decibar , centibar , and millibar... K−1 mol−1 |
|
| m3 atm K−1 mol−1 | |
| cm3 atm K−1 mol−1 | |
| m3 kgf/cm2 Kilogram-force per square centimetre A kilogram-force per square centimeter , often just kilogram per square centimeter , or kilopond per square centimeter is a unit of pressure using metric units. Its use is now deprecated; it is not a part of the International System of Units , the modern metric system... K−1 mol−1 |
|
| L bar K−1 mol−1 | |
| L mmHg K−1 mol−1 | |
| L Torr Torr The torr is a non-SI unit of pressure with the ratio of 760 to 1 standard atmosphere, chosen to be roughly equal to the fluid pressure exerted by a millimetre of mercury, i.e., a pressure of 1 torr is approximately equal to 1 mmHg... K−1 mol−1 |
|
| ft lbf Foot-pound force The foot-pound force, or simply foot-pound is a unit of work or energy in the Engineering and Gravitational Systems in United States customary and Imperial units of measure. It is the energy transferred on applying a force of 1 pound-force through a displacement of 1 foot... K−1 g-mol Mole (unit) The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value... −1 |
|
| ft lbf °R−1 lb-mol−1 | |
| ft3 Cubic foot The cubic foot is an Imperial and US customary unit of volume, used in the United States and the United Kingdom. It is defined as the volume of a cube with sides of one foot in length.-Conversions:- Symbols :... psi °R−1 lb-mol−1 |
|
| ft3 atm °R−1 lb-mol−1 | |
| ft3 atm K−1 lb-mol−1 | |
| ft3 mmHg K−1 lb-mol−1 | |
| Btu British thermal unit The British thermal unit is a traditional unit of energy equal to about 1055 joules. It is approximately the amount of energy needed to heat of water, which is exactly one tenth of a UK gallon or about 0.1198 US gallons, from 39°F to 40°F... lb-mol−1 °R−1 |
|
The gas constant (also known as the molar, universal, or ideal gas constant, denoted by the symbol R or R) is a physical constant
Physical constant
A physical constant is a physical quantity that is generally believed to be both universal in nature and constant in time. It can be contrasted with a mathematical constant, which is a fixed numerical value but does not directly involve any physical measurement.There are many physical constants in...
which is featured in many fundamental equations in the physical sciences, such as the ideal gas law
Ideal gas law
The ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. It was first stated by Émile Clapeyron in 1834 as a combination of Boyle's law and Charles's law...
and the Nernst equation
Nernst equation
In electrochemistry, the Nernst equation is an equation that can be used to determine the equilibrium reduction potential of a half-cell in an electrochemical cell. It can also be used to determine the total voltage for a full electrochemical cell...
. It is equivalent to the Boltzmann constant, but expressed in units of energy
Energy
In physics, energy is an indirectly observed quantity. It is often understood as the ability a physical system has to do work on other physical systems...
(i.e. the pressure-volume product) per temperature increment
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
per mole
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
(rather than energy per temperature increment per particle). The constant is also a combination of the constants from Boyle's Law
Boyle's law
Boyle's law is one of many gas laws and a special case of the ideal gas law. Boyle's law describes the inversely proportional relationship between the absolute pressure and volume of a gas, if the temperature is kept constant within a closed system...
, Charles' Law, Avogadro's Law
Avogadro's law
Avogadro's law is a gas law named after Amedeo Avogadro who, in 1811, hypothesized that two given samples of an ideal gas, at the same temperature, pressure and volume, contain the same number of molecules...
, and Gay-Lussac's Law
Gay-Lussac's law
The expression Gay-Lussac's law is used for each of the two relationships named after the French chemist Joseph Louis Gay-Lussac and which concern the properties of gases, though it is more usually applied to his law of combining volumes, the first listed here...
.
Its value is
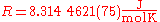
The two digits in parentheses
Bracket
Brackets are tall punctuation marks used in matched pairs within text, to set apart or interject other text. In the United States, "bracket" usually refers specifically to the "square" or "box" type.-List of types:...
are the uncertainty
Measurement uncertainty
In metrology, measurement uncertainty is a non-negative parameter characterizing the dispersion of the values attributed to a measured quantity. The uncertainty has a probabilistic basis and reflects incomplete knowledge of the quantity. All measurements are subject to uncertainty and a measured...
(standard deviation
Standard deviation
Standard deviation is a widely used measure of variability or diversity used in statistics and probability theory. It shows how much variation or "dispersion" there is from the average...
) in the last two digits of the value. The relative uncertainty is 9.1.
Some have suggested that it might be appropriate to name the symbol R as Regnault constant in honor of the French
French people
The French are a nation that share a common French culture and speak the French language as a mother tongue. Historically, the French population are descended from peoples of Celtic, Latin and Germanic origin, and are today a mixture of several ethnic groups...
chemist
Chemist
A chemist is a scientist trained in the study of chemistry. Chemists study the composition of matter and its properties such as density and acidity. Chemists carefully describe the properties they study in terms of quantities, with detail on the level of molecules and their component atoms...
, Henri Victor Regnault
Henri Victor Regnault
Henri Victor Regnault was a French chemist and physicist best known for his careful measurements of the thermal properties of gases. He was an early thermodynamicist and was mentor to William Thomson in the late 1840s....
whose accurate experimental data was used to calculate the early value of the constant, however the exact reason for the original representation of the constant by the letter R is elusive.
The gas constant occurs in the ideal gas law
Ideal gas law
The ideal gas law is the equation of state of a hypothetical ideal gas. It is a good approximation to the behavior of many gases under many conditions, although it has several limitations. It was first stated by Émile Clapeyron in 1834 as a combination of Boyle's law and Charles's law...
, as follows:
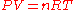
where P is the absolute pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
(SI units Pascals), V is the volume of gas (SI units m3), n is the chemical amount
Amount of substance
Amount of substance is a standards-defined quantity that measures the size of an ensemble of elementary entities, such as atoms, molecules, electrons, and other particles. It is sometimes referred to as chemical amount. The International System of Units defines the amount of substance to be...
of gas (SI units mole), and T is thermodynamic temperature
Thermodynamic temperature
Thermodynamic temperature is the absolute measure of temperature and is one of the principal parameters of thermodynamics. Thermodynamic temperature is an "absolute" scale because it is the measure of the fundamental property underlying temperature: its null or zero point, absolute zero, is the...
(SI units Kelvin). The gas constant has the same units as molar entropy
Entropy
Entropy is a thermodynamic property that can be used to determine the energy available for useful work in a thermodynamic process, such as in energy conversion devices, engines, or machines. Such devices can only be driven by convertible energy, and have a theoretical maximum efficiency when...
.
Relationship with the Boltzmann constant
The Boltzmann constant kB (often abbreviated k) may be used in place of the gas constant by working in pure particle count, N, rather than amount of substance, n, since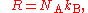
where NA is the Avogadro constant.
For example, the ideal gas law in terms of Boltzmann's constant is

where N is the number of particles (atoms or molecules).
Measurement
As of 2006, the most precise measurement of R is obtained by measuring the speed of soundSpeed of sound
The speed of sound is the distance travelled during a unit of time by a sound wave propagating through an elastic medium. In dry air at , the speed of sound is . This is , or about one kilometer in three seconds or approximately one mile in five seconds....
ca(p, T) in argon
Argon
Argon is a chemical element represented by the symbol Ar. Argon has atomic number 18 and is the third element in group 18 of the periodic table . Argon is the third most common gas in the Earth's atmosphere, at 0.93%, making it more common than carbon dioxide...
at the temperature T of the triple point of water (used to define the kelvin
Kelvin
The kelvin is a unit of measurement for temperature. It is one of the seven base units in the International System of Units and is assigned the unit symbol K. The Kelvin scale is an absolute, thermodynamic temperature scale using as its null point absolute zero, the temperature at which all...
) at different pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
s p, and extrapolating
Extrapolation
In mathematics, extrapolation is the process of constructing new data points. It is similar to the process of interpolation, which constructs new points between known points, but the results of extrapolations are often less meaningful, and are subject to greater uncertainty. It may also mean...
to the zero-pressure limit ca(0, T). The value of R is then obtained from the relation
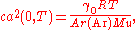
where:
- γ0 is the heat capacity ratioHeat capacity ratioThe heat capacity ratio or adiabatic index or ratio of specific heats, is the ratio of the heat capacity at constant pressure to heat capacity at constant volume . It is sometimes also known as the isentropic expansion factor and is denoted by \gamma or \kappa . The latter symbol kappa is...
(5/3 for monatomic gases such as argon); - T is the temperature, TTPW = 273.16 K by definition of the kelvin;
- Ar(Ar) is the relative atomic mass of argon and Mu = 10−3 kg mol−1.
Specific gas constant
| Rspecific for dry air |
Units |
|---|---|
| 287.04 | J kg−1 K−1 |
| 53.3533 | ft lbf Pound-force The pound force is a unit of force in some systems of measurement including English engineering units and British gravitational units.- Definitions :... lb Pound (mass) The pound or pound-mass is a unit of mass used in the Imperial, United States customary and other systems of measurement... −1 °R−1 |
| 1716.49 | ft lbf Pound-force The pound force is a unit of force in some systems of measurement including English engineering units and British gravitational units.- Definitions :... slug−1 °R−1 |
| Based on a mean molar mass for dry air of 28.9645 g/mol. |
|
The specific gas constant of a gas or a mixture of gases (Rspecific) is given by the molar gas constant, divided by the molar mass
Molar mass
Molar mass, symbol M, is a physical property of a given substance , namely its mass per amount of substance. The base SI unit for mass is the kilogram and that for amount of substance is the mole. Thus, the derived unit for molar mass is kg/mol...
(M) of the gas/mixture.

Just as the ideal gas constant can be related to the Boltzmann constant, so can the specific gas constant by dividing the Boltzmann constant by the molecular mass of the gas.

Another important relationship comes from thermodynamics. This relates the specific gas constant to the specific heats for a calorically perfect gas and a thermally perfect gas.
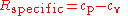
where cp is the specific heat for a constant pressure and cv is the specific heat for a constant volume.
It is common, especially in engineering applications, to represent the specific gas constant by the symbol R. In such cases, the universal gas constant is usually given a different symbol such as R to distinguish it. In any case, the context and/or units of the gas constant should make it clear as to whether the universal or specific gas constant is being referred to.
U.S. Standard Atmosphere
The U.S. Standard Atmosphere, 1976 (USSA1976) defines the gas constant R* as: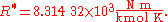
The USSA1976 does recognize, however, that this value is not consistent with the cited values for the Avogadro constant and the Boltzmann constant. This disparity is not a significant departure from accuracy, and USSA1976 uses this value of R* for all the calculations of the standard atmosphere. When using the ISO
International Organization for Standardization
The International Organization for Standardization , widely known as ISO, is an international standard-setting body composed of representatives from various national standards organizations. Founded on February 23, 1947, the organization promulgates worldwide proprietary, industrial and commercial...
value of R, the calculated pressure increases by only 0.62 pascal
Pascal (unit)
The pascal is the SI derived unit of pressure, internal pressure, stress, Young's modulus and tensile strength, named after the French mathematician, physicist, inventor, writer, and philosopher Blaise Pascal. It is a measure of force per unit area, defined as one newton per square metre...
at 11 kilometers (the equivalent of a difference of only 0.174 meter or 6.8 inches) and an increase of 0.292 Pa at 20 km (the equivalent of a difference of only 0.338 m or 13.2 in).

