
Molar mass
Encyclopedia
Molar mass, symbol M, is a physical property of a given substance (chemical element
or chemical compound
), namely its mass
per amount of substance
. The base SI unit for mass is the kilogram
and that for amount of substance is the mole
. Thus, the derived unit for molar mass is kg/mol. However, for both practical and historical reasons, molar masses are almost always quoted in grams per mole (g/mol or g mol−1), especially in chemistry
.
Molar mass is closely related to the relative molar mass (M) of a compound, the older term formula weight and to the standard atomic masses
of its constituent elements. However, it should be distinguished from the molecular mass
(also known as molecular weight), which is the mass of one molecule (of any single isotopic composition) and is not directly related to the atomic mass
, the mass of one atom (of any single isotope). The dalton, symbol Da, is also sometimes used as a unit of molar mass, especially in biochemistry
, with the definition 1 Da = 1 g/mol, despite the fact that it is strictly a unit of mass (1 Da = 1.660 538 782(83)×10−27 kg).
Molar masses are almost never measured directly. They may be calculated from standard atomic masses, and are often listed in chemical catalogues and on material safety data sheet
s (MSDS). Molar masses typically vary between:
s of an element
is given by the atomic weight
of the element multiplied by the molar mass constant
, M = 1×10−3 kg/mol = 1 g/mol:
Multiplying by the molar mass constant ensures that the calculation is dimension
ally correct: atomic weights are dimensionless quantities (i.e., pure numbers) whereas molar masses have units (in this case, grams/mole).
Some elements are usually encountered as molecule
s, e.g. hydrogen
(H), sulfur
(S), chlorine
(Cl). The molar mass of molecules of these elements is the molar mass of the atoms multiplied by the number of atoms in each molecule:
is given by the sum of the standard atomic weights of the atom
s which form the compound multiplied by the molar mass constant
, M:
An average molar mass may be defined for mixtures of compounds. This is particularly important in polymer science
, where different polymer molecules may contain different numbers of monomer
units (non-uniform polymers).
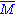 can be calculated from the mole fractions
can be calculated from the mole fractions 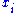 of the components and their molar masses
of the components and their molar masses  :
:

It can also be calculated from the mass fractions of the components:
of the components:
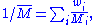
As an example, the average molar mass of dry air is 28.97 g/mol.
less quantity (i.e., a pure number, without units) equal to the molar mass divided by the molar mass constant
.
s (u), where 1 Da = 1 u = 1.660 538 782(83)×10−27 kg). Different molecules of the same compound may have different molecular masses because they contain different isotope
s of an element. The molar mass is a measure of the average molecular mass of all the molecules in a sample, and is usually the more appropriate measure when dealing with macroscopic (weighable) quantities of a substance.
Molecular masses are calculated from the relative atomic masses of each nuclide
, while molar masses are calculated from the atomic weight
s of each element
. The atomic weight takes into account the isotopic distribution
of the element in a given sample (usually assumed to be "normal"). For example, water has a molar mass of 18.0153(3) g/mol, but individual water molecules have molecular masses which range between 18.010 564 6863(15) u (1H16O) and 22.027 7364(9) u (D18O).
The distinction between molar mass and molecular mass is important because relative molecular masses can be measured directly by mass spectrometry
, often to a precision of a few parts per million. This is accurate enough to directly determine the chemical formula
of a molecule.
nucleobase to be added to a DNA polymer has protecting groups and has its molecular weight quoted including these groups, the amount of molecular weight that is ultimately added by this nucleobase to a DNA polymer is referred to as the nucleobase's formula weight (i.e., the molecular weight of this nucleobase within the DNA polymer, minus protecting groups).
s from which it was calculated. Most atomic weights are known to a precision of at least one part in ten-thousand, often much better (the atomic weight of lithium
is a notable, and serious, exception). This is adequate for almost all normal uses in chemistry: it is more precise than most chemical analyses, and exceeds the purity of most laboratory reagents.
The precision of atomics weights, and hence of molar masses, is limited by the knowledge of the isotopic distribution
of the element. If a more accurate value of the molar mass is required, it is necessary to determine the isotopic distribution of the sample in question, which may be different from the standard distribution used to calculate the standard atomic weight. The isotopic distributions of the different elements in a sample are not necessarily independent of one another: for example, a sample which has been distilled
will be enriched in the lighter isotope
s of all the elements present. This complicates the calculation of the standard uncertainty in the molar mass.
A useful convention for normal laboratory work is to quote molar masses to two decimal places for all calculations. This is more accurate than is usually required, but avoids rounding errors during calculations. When the molar mass is greater than 1000 g/mol, it is rarely appropriate to use more than one decimal place. These conventions are followed in most tabulated values of molar masses.
measurements of atomic weights and molecular masses, and are of mostly historical interest. All of the procedures rely on colligative properties, and any dissociation
of the compound must be taken into account.
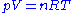
where n is the amount of substance
. The vapour density (ρ) is given by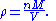
Combining these two equations gives an expression for the molar mass in terms of the vapour density for conditions of known pressure
and temperature
.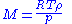
of a solution
is lower than that of the pure solvent
, and the freezing-point depression (ΔT) is directly proportional to the amount concentration for dilute solutions. When the amount concentration is expressed as a molality, the proportionality constant is known as the cryoscopic constant
(K) and is characteristic for each solvent. If w represents the mass fraction of the solute
in solution, and assuming no dissociation of the solute, the molar mass is given by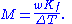
of a solution
of an involatile solute
is higher than that of the pure solvent
, and the boiling-point elevation (ΔT) is directly proportional to the amount concentration for dilute solutions. When the amount concentration is expressed as a molality, the proportionality constant is known as the ebullioscopic constant
(K) and is characteristic for each solvent. If w represents the mass fraction of the solute in solution, and assuming no dissociation of the solute, the molar mass is given by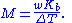
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
or chemical compound
Chemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
), namely its mass
Mass
Mass can be defined as a quantitive measure of the resistance an object has to change in its velocity.In physics, mass commonly refers to any of the following three properties of matter, which have been shown experimentally to be equivalent:...
per amount of substance
Amount of substance
Amount of substance is a standards-defined quantity that measures the size of an ensemble of elementary entities, such as atoms, molecules, electrons, and other particles. It is sometimes referred to as chemical amount. The International System of Units defines the amount of substance to be...
. The base SI unit for mass is the kilogram
Kilogram
The kilogram or kilogramme , also known as the kilo, is the base unit of mass in the International System of Units and is defined as being equal to the mass of the International Prototype Kilogram , which is almost exactly equal to the mass of one liter of water...
and that for amount of substance is the mole
Mole (unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities as there are atoms in 12 grams of pure carbon-12 , the isotope of carbon with atomic weight 12. This corresponds to a value...
. Thus, the derived unit for molar mass is kg/mol. However, for both practical and historical reasons, molar masses are almost always quoted in grams per mole (g/mol or g mol−1), especially in chemistry
Chemistry
Chemistry is the science of matter, especially its chemical reactions, but also its composition, structure and properties. Chemistry is concerned with atoms and their interactions with other atoms, and particularly with the properties of chemical bonds....
.
Molar mass is closely related to the relative molar mass (M) of a compound, the older term formula weight and to the standard atomic masses
Atomic mass
The atomic mass is the mass of a specific isotope, most often expressed in unified atomic mass units. The atomic mass is the total mass of protons, neutrons and electrons in a single atom....
of its constituent elements. However, it should be distinguished from the molecular mass
Molecular mass
The molecular mass of a substance is the mass of one molecule of that substance, in unified atomic mass unit u...
(also known as molecular weight), which is the mass of one molecule (of any single isotopic composition) and is not directly related to the atomic mass
Atomic mass
The atomic mass is the mass of a specific isotope, most often expressed in unified atomic mass units. The atomic mass is the total mass of protons, neutrons and electrons in a single atom....
, the mass of one atom (of any single isotope). The dalton, symbol Da, is also sometimes used as a unit of molar mass, especially in biochemistry
Biochemistry
Biochemistry, sometimes called biological chemistry, is the study of chemical processes in living organisms, including, but not limited to, living matter. Biochemistry governs all living organisms and living processes...
, with the definition 1 Da = 1 g/mol, despite the fact that it is strictly a unit of mass (1 Da = 1.660 538 782(83)×10−27 kg).
Molar masses are almost never measured directly. They may be calculated from standard atomic masses, and are often listed in chemical catalogues and on material safety data sheet
Material safety data sheet
A Material Safety Data Sheet is a form with data regarding the properties of a particular substance....
s (MSDS). Molar masses typically vary between:
- 1–238 g/mol for atoms of naturally-occurring elements;
- 10–1000 g/mol for simple chemical compoundsSmall moleculeIn the fields of pharmacology and biochemistry, a small molecule is a low molecular weight organic compound which is by definition not a polymer...
; - 1000–5,000,000 g/mol for polymerPolymerA polymer is a large molecule composed of repeating structural units. These subunits are typically connected by covalent chemical bonds...
s, proteinProteinProteins are biochemical compounds consisting of one or more polypeptides typically folded into a globular or fibrous form, facilitating a biological function. A polypeptide is a single linear polymer chain of amino acids bonded together by peptide bonds between the carboxyl and amino groups of...
s, DNADNADeoxyribonucleic acid is a nucleic acid that contains the genetic instructions used in the development and functioning of all known living organisms . The DNA segments that carry this genetic information are called genes, but other DNA sequences have structural purposes, or are involved in...
fragments, etc.
Molar masses of elements
The molar mass of atomAtom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s of an element
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
is given by the atomic weight
Atomic weight
Atomic weight is a dimensionless physical quantity, the ratio of the average mass of atoms of an element to 1/12 of the mass of an atom of carbon-12...
of the element multiplied by the molar mass constant
Molar mass constant
The molar mass constant, symbol Mu, is a physical constant which relates atomic weight and molar mass. Its value is defined to be 1 g/mol in SI units....
, M = 1×10−3 kg/mol = 1 g/mol:
- M(H) = 1.007 97(7) × 1 g/mol = 1.007 97(7) g/mol
- M(S) = 32.065(5) × 1 g/mol = 32.065(5) g/mol
- M(Cl) = 35.453(2) × 1 g/mol = 35.453(2) g/mol
- M(Fe) = 55.845(2) × 1 g/mol = 55.845(2) g/mol.
Multiplying by the molar mass constant ensures that the calculation is dimension
Dimension
In physics and mathematics, the dimension of a space or object is informally defined as the minimum number of coordinates needed to specify any point within it. Thus a line has a dimension of one because only one coordinate is needed to specify a point on it...
ally correct: atomic weights are dimensionless quantities (i.e., pure numbers) whereas molar masses have units (in this case, grams/mole).
Some elements are usually encountered as molecule
Molecule
A molecule is an electrically neutral group of at least two atoms held together by covalent chemical bonds. Molecules are distinguished from ions by their electrical charge...
s, e.g. hydrogen
Hydrogen
Hydrogen is the chemical element with atomic number 1. It is represented by the symbol H. With an average atomic weight of , hydrogen is the lightest and most abundant chemical element, constituting roughly 75% of the Universe's chemical elemental mass. Stars in the main sequence are mainly...
(H), sulfur
Sulfur
Sulfur or sulphur is the chemical element with atomic number 16. In the periodic table it is represented by the symbol S. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow...
(S), chlorine
Chlorine
Chlorine is the chemical element with atomic number 17 and symbol Cl. It is the second lightest halogen, found in the periodic table in group 17. The element forms diatomic molecules under standard conditions, called dichlorine...
(Cl). The molar mass of molecules of these elements is the molar mass of the atoms multiplied by the number of atoms in each molecule:
- M(H) = 2 × 1.007 97(7) × 1 g/mol = 2.015 88(14) g/mol
- M(S) = 8 × 32.065(5) × 1 g/mol = 256.52(4) g/mol
- M(Cl) = 2 × 35.453(2) × 1 g/mol = 70.906(4) g/mol.
Molar masses of compounds
The molar mass of a compoundChemical compound
A chemical compound is a pure chemical substance consisting of two or more different chemical elements that can be separated into simpler substances by chemical reactions. Chemical compounds have a unique and defined chemical structure; they consist of a fixed ratio of atoms that are held together...
is given by the sum of the standard atomic weights of the atom
Atom
The atom is a basic unit of matter that consists of a dense central nucleus surrounded by a cloud of negatively charged electrons. The atomic nucleus contains a mix of positively charged protons and electrically neutral neutrons...
s which form the compound multiplied by the molar mass constant
Molar mass constant
The molar mass constant, symbol Mu, is a physical constant which relates atomic weight and molar mass. Its value is defined to be 1 g/mol in SI units....
, M:
- M(NaCl) = [22.989 769 28(2) + 35.453(2)] × 1 g/mol = 58.443(2) g/mol
- M(CHO) = ([12 × 12.0107(8)] + [22 ×1.007 94(7)] + [11 ×15.9994(3)]) × 1 g/mol = 342.297(14) g/mol.
An average molar mass may be defined for mixtures of compounds. This is particularly important in polymer science
Polymer science
Polymer science or macromolecular science is the subfield of materials science concerned with polymers, primarily synthetic polymers such as plastics...
, where different polymer molecules may contain different numbers of monomer
Monomer
A monomer is an atom or a small molecule that may bind chemically to other monomers to form a polymer; the term "monomeric protein" may also be used to describe one of the proteins making up a multiprotein complex...
units (non-uniform polymers).
Average molar mass of mixtures
The average molar mass of mixtures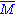 can be calculated from the mole fractions
can be calculated from the mole fractions 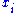 of the components and their molar masses
of the components and their molar masses  :
:
It can also be calculated from the mass fractions
 of the components:
of the components: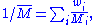
As an example, the average molar mass of dry air is 28.97 g/mol.
Related properties
Molecular weight (M.W.) and formula weight (F.W.) are older terms for what is now more correctly called the relative molar mass (M). This is a dimensionDimension
In physics and mathematics, the dimension of a space or object is informally defined as the minimum number of coordinates needed to specify any point within it. Thus a line has a dimension of one because only one coordinate is needed to specify a point on it...
less quantity (i.e., a pure number, without units) equal to the molar mass divided by the molar mass constant
Molar mass constant
The molar mass constant, symbol Mu, is a physical constant which relates atomic weight and molar mass. Its value is defined to be 1 g/mol in SI units....
.
Molecular mass
The molecular mass (m) is the mass of a given molecule: it is measured in daltons (Da) or atomic mass unitAtomic mass unit
The unified atomic mass unit or dalton is a unit that is used for indicating mass on an atomic or molecular scale. It is defined as one twelfth of the rest mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state, and has a value of...
s (u), where 1 Da = 1 u = 1.660 538 782(83)×10−27 kg). Different molecules of the same compound may have different molecular masses because they contain different isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
s of an element. The molar mass is a measure of the average molecular mass of all the molecules in a sample, and is usually the more appropriate measure when dealing with macroscopic (weighable) quantities of a substance.
Molecular masses are calculated from the relative atomic masses of each nuclide
Nuclide
A nuclide is an atomic species characterized by the specific constitution of its nucleus, i.e., by its number of protons Z, its number of neutrons N, and its nuclear energy state....
, while molar masses are calculated from the atomic weight
Atomic weight
Atomic weight is a dimensionless physical quantity, the ratio of the average mass of atoms of an element to 1/12 of the mass of an atom of carbon-12...
s of each element
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
. The atomic weight takes into account the isotopic distribution
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
of the element in a given sample (usually assumed to be "normal"). For example, water has a molar mass of 18.0153(3) g/mol, but individual water molecules have molecular masses which range between 18.010 564 6863(15) u (1H16O) and 22.027 7364(9) u (D18O).
The distinction between molar mass and molecular mass is important because relative molecular masses can be measured directly by mass spectrometry
Mass spectrometry
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
, often to a precision of a few parts per million. This is accurate enough to directly determine the chemical formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
of a molecule.
DNA synthesis usage
The term formula weight (F.W.) has a specific meaning when used in the context of DNA synthesis: whereas an individual phosphoramiditePhosphoramidite
Nucleoside phosphoramidites are derivatives of natural or synthetic nucleosides. They are used to synthesize oligonucleotides, relatively short fragments of nucleic acid and their analogs. Nucleoside phosphoramidites were first introduced in 1981 by Beaucage and Caruthers...
nucleobase to be added to a DNA polymer has protecting groups and has its molecular weight quoted including these groups, the amount of molecular weight that is ultimately added by this nucleobase to a DNA polymer is referred to as the nucleobase's formula weight (i.e., the molecular weight of this nucleobase within the DNA polymer, minus protecting groups).
Precision and uncertainties
The precision to which a molar mass is known depends on the precision of the atomic weightAtomic weight
Atomic weight is a dimensionless physical quantity, the ratio of the average mass of atoms of an element to 1/12 of the mass of an atom of carbon-12...
s from which it was calculated. Most atomic weights are known to a precision of at least one part in ten-thousand, often much better (the atomic weight of lithium
Lithium
Lithium is a soft, silver-white metal that belongs to the alkali metal group of chemical elements. It is represented by the symbol Li, and it has the atomic number 3. Under standard conditions it is the lightest metal and the least dense solid element. Like all alkali metals, lithium is highly...
is a notable, and serious, exception). This is adequate for almost all normal uses in chemistry: it is more precise than most chemical analyses, and exceeds the purity of most laboratory reagents.
The precision of atomics weights, and hence of molar masses, is limited by the knowledge of the isotopic distribution
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
of the element. If a more accurate value of the molar mass is required, it is necessary to determine the isotopic distribution of the sample in question, which may be different from the standard distribution used to calculate the standard atomic weight. The isotopic distributions of the different elements in a sample are not necessarily independent of one another: for example, a sample which has been distilled
Distillation
Distillation is a method of separating mixtures based on differences in volatilities of components in a boiling liquid mixture. Distillation is a unit operation, or a physical separation process, and not a chemical reaction....
will be enriched in the lighter isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
s of all the elements present. This complicates the calculation of the standard uncertainty in the molar mass.
A useful convention for normal laboratory work is to quote molar masses to two decimal places for all calculations. This is more accurate than is usually required, but avoids rounding errors during calculations. When the molar mass is greater than 1000 g/mol, it is rarely appropriate to use more than one decimal place. These conventions are followed in most tabulated values of molar masses.
Measurement
While molar masses are almost always, in practice, calculated from atomic weights, they can also be measured in certain cases. Such measurements are much less precise than modern mass spectrometricMass spectrometry
Mass spectrometry is an analytical technique that measures the mass-to-charge ratio of charged particles.It is used for determining masses of particles, for determining the elemental composition of a sample or molecule, and for elucidating the chemical structures of molecules, such as peptides and...
measurements of atomic weights and molecular masses, and are of mostly historical interest. All of the procedures rely on colligative properties, and any dissociation
Dissociation
Dissociation is an altered state of consciousness characterized by partial or complete disruption of the normal integration of a person’s normal conscious or psychological functioning. Dissociation is most commonly experienced as a subjective perception of one's consciousness being detached from...
of the compound must be taken into account.
Vapour density
The measurement of molar mass by vapour density relies on the principle, first enounced by Amadeo Avogadro, that equal volumes of gases under identical conditions contain equal numbers of particles. This principle is included in the ideal gas equation: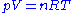
where n is the amount of substance
Amount of substance
Amount of substance is a standards-defined quantity that measures the size of an ensemble of elementary entities, such as atoms, molecules, electrons, and other particles. It is sometimes referred to as chemical amount. The International System of Units defines the amount of substance to be...
. The vapour density (ρ) is given by
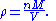
Combining these two equations gives an expression for the molar mass in terms of the vapour density for conditions of known pressure
Pressure
Pressure is the force per unit area applied in a direction perpendicular to the surface of an object. Gauge pressure is the pressure relative to the local atmospheric or ambient pressure.- Definition :...
and temperature
Temperature
Temperature is a physical property of matter that quantitatively expresses the common notions of hot and cold. Objects of low temperature are cold, while various degrees of higher temperatures are referred to as warm or hot...
.
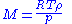
Freezing-point depression
The freezing pointFreezing Point
Freezing Point is a news journal in the People's Republic of China which has been the subject of controversy over its criticism of Communist Party officials and the sympathetic ear it lent to a Chinese historian who had criticized official history textbooks...
of a solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
is lower than that of the pure solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
, and the freezing-point depression (ΔT) is directly proportional to the amount concentration for dilute solutions. When the amount concentration is expressed as a molality, the proportionality constant is known as the cryoscopic constant
Cryoscopic constant
In thermodynamics, the cryoscopic constant, Kf, allows one to relate molality to freezing point depression. It is the ratio of the latter to the former:\triangle T_f = K_f \cdot m \cdot i...
(K) and is characteristic for each solvent. If w represents the mass fraction of the solute
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
in solution, and assuming no dissociation of the solute, the molar mass is given by
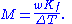
Boiling-point elevation
The boiling pointBoiling point
The boiling point of an element or a substance is the temperature at which the vapor pressure of the liquid equals the environmental pressure surrounding the liquid....
of a solution
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
of an involatile solute
Solution
In chemistry, a solution is a homogeneous mixture composed of only one phase. In such a mixture, a solute is dissolved in another substance, known as a solvent. The solvent does the dissolving.- Types of solutions :...
is higher than that of the pure solvent
Solvent
A solvent is a liquid, solid, or gas that dissolves another solid, liquid, or gaseous solute, resulting in a solution that is soluble in a certain volume of solvent at a specified temperature...
, and the boiling-point elevation (ΔT) is directly proportional to the amount concentration for dilute solutions. When the amount concentration is expressed as a molality, the proportionality constant is known as the ebullioscopic constant
Ebullioscopic constant
In thermodynamics, the ebullioscopic constant, Kb, allows one to relate molality to boiling point elevation. It is the ratio of the latter to the former:\Delta T = i\cdot K_b \cdot mi is the Vant Hoff factor...
(K) and is characteristic for each solvent. If w represents the mass fraction of the solute in solution, and assuming no dissociation of the solute, the molar mass is given by
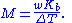
External links
- Online Molar Mass Calculator with the uncertainty of M and all the calculations shown
- Molar Mass Calculator Online Molar Mass and Elemental Composition Calculator
- Stoichiometry Add-In for Microsoft Excel for calculation of molecular weights, reaction coëfficients and stoichiometry. It includes both average atomic weights and isotopic weights.
- Mass and Formulae tools Formula to mass and mass to formulae web tools

