
Lead
Encyclopedia
Lead is a main-group element
in the carbon group
with the symbol Pb (from ) and atomic number
82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed to air. Lead has a shiny chrome-silver luster when it is melted into a liquid.
Lead is used in building construction, lead-acid batteries, bullet
s and shot
s, weights, as part of solder
s, pewter
s, fusible alloy
s and as a radiation shield. Lead has the highest atomic number
of all of the stable elements, although the next higher element, bismuth
, has a half-life
that is so long (much longer than the age of the universe) that it can be considered stable. Its four stable isotopes have 82 proton
s, a magic number
in the nuclear shell model of atomic nuclei.
Lead, at certain exposure levels, is a poisonous substance to animals as well as for human beings. It damages the nervous system
and causes brain
disorders. Excessive lead also causes blood disorders in mammals. Like the element mercury
, another heavy metal, lead is a neurotoxin
that accumulates both in soft tissues and the bones. Lead poisoning
has been documented from ancient Rome
, ancient Greece
, and ancient China.
, ductile, very soft, highly malleable, bluish-white metal that has poor electrical conductivity when compared to most other metals. This metal is highly resistant to corrosion
, and because of this property, it is used to contain corrosive liquids (for example, sulfuric acid
). Because lead is very malleable and resistant to corrosion it is extensively used in building construction – for example in the external coverings of roofing joints.
Metallic lead can be toughened by addition of small amounts of antimony
, or a small number of other metals such as calcium
. All isotope
s of lead, except for lead-204, can be found in the end products of the radioactive decay
of the even heavier elements, uranium
and thorium
.
Powdered lead burns with a bluish-white flame. As with many metals, finely divided powdered lead exhibits pyrophoricity
. Toxic fumes are released when lead is burned.
s, with three of them being stable. The four natural isotopes of lead are 204Pb, 206Pb, 207Pb, and 208Pb with the slightly radioactive 204Pb regarded as completely primordial lead, and the stable isotopes 206, 207, 208 being formed probably from the radioactive decay
of two isotopes of uranium (U-235
and U-238
) and one isotope of thorium
(Th 232).
The one common radiogenic isotope of lead, 202Pb,it has a half-life
of about 53,000 years.
Metallic lead is attacked (oxidized) only superficially by air, forming a thin layer of lead oxide that protects it from further oxidation. The metal is not attacked by sulfuric
or hydrochloric
acids. It dissolves in nitric acid
with the evolution of nitric oxide
gas to form dissolved Pb(NO3)2
.
When heated with nitrate
s of alkali metals, metallic lead oxidizes to form PbO
(also known as litharge
), leaving the corresponding alkali nitrite
. PbO is representative of lead's +2 oxidation state. It is soluble in nitric
and acetic
acids, from which solutions it is possible to precipitate halide
, sulfate, chromate, carbonate
(PbCO3), and basic carbonate ( salts of lead. The sulfide
can also be precipitated from acetate
solutions. These salts are all poorly soluble in water. Among the halides, the iodide is less soluble than the bromide, which, in turn, is less soluble than the chloride.
Lead(II) oxide is also soluble in alkali
hydroxide
solutions to form the corresponding plumbite
salt.
Chlorination
of plumbite solutions causes the formation of lead's +4 oxidation state.
Lead dioxide is representative of the +4 oxidation state, and is a powerful oxidizing agent
. The chloride of this oxidation state is formed only with difficulty and decomposes readily into lead(II) chloride and chlorine gas. The bromide and iodide of lead(IV) are not known to exist. Lead dioxide dissolves in alkali hydroxide solutions to form the corresponding plumbate
s.
Lead also has an oxide with mixed +2 and +4 oxidation states, red lead , also known as minium.
Lead readily forms an equimolar alloy with sodium
metal that reacts with alkyl halides to form organometallic compounds of lead such as tetraethyllead.
 Lead(II) forms a series of complexes with chloride
Lead(II) forms a series of complexes with chloride
, the formation of which alters the corrosion chemistry of the lead. This will tend to limit the solubility of lead in saline
media.
The addition of chloride can lower the solubility of lead, though in chloride-rich media (such as aqua regia
) the lead can become soluble again as anionic chloro-complexes.
. Metallic lead beads dating back to 6400 BCE have been found in Çatalhöyük
in modern-day Turkey. In the early Bronze Age
, lead was used with antimony
and arsenic
.
The largest preindustrial
producer of lead was the Roman economy
, with an estimated output per annum of 80,000 t, which was typically won as a by-product of extensive silver smelting. Roman mining activities
occurred in Central Europe
, Roman Britain
, the Balkans
, Greece
, Asia Minor
; Hispania
alone accounted for 40% of world production.
Roman lead pipes often bore the insignia of Roman emperors (see Roman lead pipe inscription
s). Lead plumbing in the Latin West may have been continued beyond the age of Theoderic the Great into the medieval period. Many Roman "pigs" (ingots) of lead figure in Derbyshire lead mining history
and in the history of the industry in other English centers. The Romans also used lead in molten form to secure iron pins that held together large limestone
blocks in certain monumental buildings. In alchemy
, lead was thought to be the oldest metal and was associated with the planet Saturn
. Alchemists accordingly used Saturn's symbol (the scythe) to refer to lead.
Lead's symbol Pb is an abbreviation of its Latin
name plumbum for soft metals; originally it was plumbum nigrum (literally, "black plumbum"), where plumbum candidum (literally, "bright plumbum") was tin
. The English words "plumbing
", "plumber
", "plumb", and "plumb-bob
" also derive from this Latin root.
with zinc
, silver
and (most abundantly) copper
, and is extracted together with these metals. The main lead mineral
is galena
(PbS), which contains 86.6 % lead by weight. Other common varieties are cerussite
(PbCO3) and anglesite
(PbSO4).
typically to 70% or more. Sulfide
ores are roasted
, producing primarily lead oxide and a mixture of sulfates and silicates of lead and other metals contained in the ore.
Lead oxide from the roasting process is reduced in a coke-fired blast furnace
. This converts most of the lead to its metallic form. Three additional layers separate in the process and float to the top of the metallic lead. These are slag
(silicates containing 1.5% lead), matte
(sulfides containing 15% lead), and speiss
(arsenides of iron and copper). These wastes contain concentrations of copper, zinc, cadmium, and bismuth that can be recovered economically, as can their content of unreduced lead.
Metallic lead that results from the roasting and blast furnace processes still contains significant contaminants of arsenic, antimony, bismuth, zinc, copper, silver, and gold. The melt is treated in a reverberatory furnace
with air, steam, and sulfur, which oxidizes the contaminants except silver, gold, and bismuth. The oxidized contaminants are removed by dross
ing, where they float to the top and are skimmed off.
Most lead ores contain significant concentrations of silver
, resulting in the smelted metal also containing silver as a contaminant. Metallic silver as well as gold is removed and recovered economically by means of the Parkes process
.
Desilvered lead is freed of bismuth
according to the Betterton-Kroll process
by treating it with metallic calcium and magnesium, which forms a bismuth dross that can be skimmed off.
Very pure lead can be obtained by processing smelted lead electrolytically by means of the Betts process
. The process uses anodes of impure lead and cathodes of pure lead in an electrolyte of silica fluoride.
, 9.6 million tonnes of lead were produced, of which 4.1 million tonnes came from mining.
At current use rates, the supply of lead is estimated to run out in 42 years. Environmental analyst Lester Brown has suggested lead could run out within 18 years based on an extrapolation of 2% growth per year. This may need to be reviewed to take account of renewed interest in recycling
, and rapid progress in fuel cell
technology. According to the International Resource Panel
's Metal Stocks in Society report
, the global per capita stock of lead in use in society is 8 kg. Much of this is in more-developed countries (20–150 kg per capita) rather than less-developed countries (1–4 kg per capita).
of 22.2 years, the radioactive isotope 210Pb
is used for dating material from marine sediment
cores by radiometric
methods.
 Because of its high density and resistance from corrosion, lead is used for the ballast
Because of its high density and resistance from corrosion, lead is used for the ballast
keel of sailboats. Its high density allows it to counterbalance the heeling effect of wind on the sails while at the same time occupying a small volume and thus offering the least underwater resistance. For the same reason it is used in scuba diving
weight belts
to counteract the diver's natural buoyancy and that of his equipment. It does not have the weight-to-volume ratio of many heavy metals, but its low cost increases its use in these and other applications.

 Lead is used in applications where its low melting point, ductility and high density is an advantage. The low melting point makes casting of lead easy, and therefore small arms ammunition and shotgun pellets can be cast with minimal technical equipment. It is also inexpensive and denser than other common metals. The hot metal typesetting
Lead is used in applications where its low melting point, ductility and high density is an advantage. The low melting point makes casting of lead easy, and therefore small arms ammunition and shotgun pellets can be cast with minimal technical equipment. It is also inexpensive and denser than other common metals. The hot metal typesetting
uses a lead based alloy to produce the types for printing directly before printing.
Its corrosion resistance makes it suitable for outdoor applications when in contact with water.
More than half of the worldwide lead production (at least 1.15 million metric tons) is used for automobiles, mostly as electrodes in the lead–acid battery, used extensively as a car battery
.
Cathode
(reduction
)
Anode
(oxidation)
Lead is used as electrodes in the process of electrolysis
. Lead is used in solder
for electronics, although this usage is being phased out by some countries to reduce the amount of environmentally hazardous waste. Lead is used in high voltage power cables as sheathing material to prevent water diffusion into insulation.
Lead is one of three metals used in the Oddy test
for museum materials, helping detect organic acids, aldehydes, and acidic gases.
Lead is used as shielding
from radiation
(e.g., in X-ray
rooms). Molten lead is used as a coolant
(e.g., for lead cooled fast reactor
s).
Lead is added to brass
to reduce machine tool
wear.
Lead, in the form of strips, or tape, is used for the customization of tennis rackets. Tennis rackets of the past sometimes had lead added to them by the manufacturer to increase weight.
Lead is used to form glazing bars for stained glass
or other multi-lit windows. The practice has become less common, not for danger but for stylistic reasons.
Lead, or sheet-lead, is used as a sound deadening layer in some areas in wall, floor and ceiling design in sound studios where levels of airborne and mechanically produced sound are targeted for reduction or virtual elimination.
Lead is the traditional base metal of organ pipe
s, mixed with varying amounts of tin
to control the tone of the pipe.
Lead has many uses in the construction industry (e.g., lead sheets are used as architectural metals
in roofing material, cladding, flashing, gutters and gutter joints, and on roof parapets). Detailed lead moldings are used as decorative motifs used to fix lead sheet. Lead is still widely used in statues and sculptures.
Lead is often used to balance
the wheels of a car; this use is being phased out in favor of other materials for environmental reasons.
s, notably in the colors red and yellow.
Lead is frequently used in polyvinyl chloride
(PVC) plastic, which coats electrical cords.
Lead is used in some candles to treat the wick to ensure a longer, more even burn. Because of the dangers, European and North American manufacturers use more expensive alternatives such as zinc. Lead glass
is composed of 12–28% lead oxide
. It changes the optical characteristics of the glass and reduces the transmission of radiation.
Some artists using oil-based paints continue to use lead carbonate white, citing its properties in comparison with the alternatives. Tetra-ethyl lead is used as an anti-knock additive for aviation fuel in piston-driven aircraft. Lead-based semiconductors, such as lead telluride, lead selenide and lead antimonide are finding applications in photovoltaic (solar energy) cells and infrared
detectors.
for white as well as yellow
, orange, and red. Most uses have been discontinued due of the dangers of lead poisoning. Beginning April 22, 2010, US federal law requires that contractors performing renovation, repair, and painting projects that disturb more than six square feet of paint in homes, child care facilities, and schools built before 1978 must be certified and trained to follow specific work practices to prevent lead contamination. Lead chromate is still in industrial use. Lead carbonate (white) is the traditional pigment for the priming medium for oil painting, but it has been largely displaced by the zinc and titanium oxide pigments. It was also quickly replaced in water-based painting mediums. Lead carbonate white was used by the Japanese geisha
and in the West for face-whitening make-up, which was detrimental to health.
Lead is the hot metal that was used in hot metal typesetting
. It was used for plumbing
as well as a preservative
for food and drink in Ancient Rome
. Until the early 1970s, lead was used for joining cast iron water pipes and used as a material for small diameter water pipes.
Tetraethyllead was used in leaded fuels to reduce engine knocking
, but this practice has been phased out across many countries of the world in efforts to reduce toxic pollution that affected humans and the environment.
Lead was used to make bullets for slings
. Lead was used for shotgun
pellets in the US until about 1992 when it was outlawed (for waterfowl
hunting only) and replaced by non-toxic shot, primarily steel pellets. In the Netherlands
, the use of lead shot for hunting and sport shooting was banned in 1993, which caused a large drop in lead emission, from 230 ton in 1990 to 47.5 ton in 1995, two years after the ban.
Lead was a component of the paint used on children's toys – now restricted in the United States and across Europe (ROHS Directive). Lead was used in car body filler, which was used in many custom car
s in the 1940s–60s. Hence the term Leadsled. Lead is a superconductor at 7.2 K and IBM
tried to make a Josephson effect
computer out of lead-alloy.
Lead was also used in pesticides before the 1950s, when fruit orchards were treated (ATSDR). A lead cylinder attached to a long line was used by sailors for the vital navigational task of determining water depth by heaving the lead at regular internals. A soft tallow insert at its base allowed the nature of the sea bed to be determined, further aiding position finding. Contrary to popular belief, pencil leads in wooden pencils have never been made from lead. The term comes from the Roman stylus, called the penicillus, which was made of lead without a wooden holder. When the pencil originated as a wrapped graphite writing tool, the particular type of graphite
being used was named plumbago (lit. act for lead, or lead mockup).
, and colic
-like abdominal pains. The effects of lead are the same whether it enters the body through breathing or swallowing. Lead can affect almost every organ and system in the body. The main target for lead toxicity is the nervous system, both in adults and children. Long-term exposure of adults can result in decreased performance in some tests that measure functions of the nervous system. It may also cause weakness in fingers, wrists, or ankles. Lead exposure also causes small increases in blood pressure, particularly in middle-aged and older people and can cause anemia. Exposure to high lead levels can severely damage the brain and kidneys in adults or children and ultimately cause death. In pregnant women, high levels of exposure to lead may cause miscarriage. Chronic, high-level exposure have shown to reduce fertility in males. The antidote/treatment for lead poisoning consists of dimercaprol
and succimer.
The concern about lead's role in cognitive deficits in children has brought about widespread reduction in its use (lead exposure has been linked to learning disabilities). Most cases of adult elevated blood lead levels are workplace-related. High blood levels are associated with delayed puberty in girls. Lead has been shown many times to permanently reduce the cognitive capacity of children at extremely low levels of exposure.
During the 20th century, the use of lead in paint pigment
s was sharply reduced because of the danger of lead poisoning, especially to children. By the mid-1980s, a significant shift in lead end-use patterns had taken place. Much of this shift was a result of the U.S. lead consumers' compliance with environmental regulations that significantly reduced or eliminated the use of lead in non-battery products, including gasoline
, paints, solders, and water systems. Lead use is being further curtailed by the European Union's RoHS directive
. Lead may still be found in harmful quantities in stoneware, vinyl (such as that used for tubing and the insulation of electrical cords), and brass manufactured in China. Between 2006 and 2007 many children's toys made in China were recalled, primarily due to lead in paint used to color the product.
Older houses may still contain substantial amounts of lead paint
. White lead paint has been withdrawn from sale in industrialized countries, but the yellow lead chromate is still in use; for example, Holland Colours Holcolan Yellow. Old paint should not be stripped by sanding, as this produces inhalable dust.
Lead salts used in pottery glazes have on occasion caused poisoning, when acidic drinks, such as fruit juices, have leached lead ions out of the glaze. It has been suggested that what was known as "Devon colic
" arose from the use of lead-lined presses to extract apple juice in the manufacture of cider
. Lead is considered to be particularly harmful for women's ability to reproduce. Lead(II) acetate
(also known as sugar of lead) was used by the Roman Empire
as a sweetener for wine, and some consider this to be the cause of the dementia
that affected many of the Roman Emperors.
Lead as a soil contaminant is a widespread issue, since lead is present in natural deposits and may also enter soil through (leaded) gasoline leaks from underground storage tank
s or through a wastestream of lead paint or lead grindings from certain industrial operations.
Lead can also be found listed as a criteria pollutant in the United States Clean Air Act section 108. Lead that is emitted into the atmosphere can be inhaled, or it can be ingested after it settles out of the air. It is rapidly absorbed into the bloodstream and is believed to have adverse effects on the central nervous system, the cardiovascular system, kidneys, and the immune system.
and ferrochelatase
, preventing both porphobilinogen
formation and the incorporation of iron
into protoporphyrin IX
, the final step in heme
synthesis. This causes ineffective heme synthesis and subsequent microcytic anemia
. At lower levels, it acts as a calcium analog, interfering with ion channels during nerve conduction. This is one of the mechanisms by which it interferes with cognition. Acute lead poisoning is treated using disodium calcium edetate: the calcium chelate of the disodium salt of ethylene-diamine-tetracetic acid (EDTA
). This chelating agent has a greater affinity for lead than for calcium and so the lead chelate is formed by exchange. This is then excreted in the urine leaving behind harmless calcium.
below shows that lead is more likely to corrode in a citrate medium than it is in a non-complexing medium. The central part of the diagram shows that lead metal oxidizes more easily in the citrate medium than in normal water.
In a Pourbaix diagram, the acidity is plotted on the x axis using the pH scale, while how oxidizing/reducing nature of the system is plotted on the y axis in terms of volts relative to the standard hydrogen electrode
. The diagram shows the form of the element which is most chemically stable at each point, it only comments on thermodynamics
and it says nothing about the rate of change (kinetics
).
Lead exposure mostly occurs through ingestion. Lead paint is the major source of lead exposure for children. As lead paint deteriorates, it peels, is pulverized into dust and then enters the body through hand-to-mouth contact or through contaminated food, water or alcohol. Ingesting certain home remedy medicines may also expose people to lead or lead compounds. Lead can be ingested through fruits and vegetables contaminated by high levels of lead in the soils they were grown in. Soil is contaminated through particulate accumulation from lead in pipes, lead paint and residual emissions from leaded gasoline that was used before the Environment Protection Agency issue the regulation around 1980.
Inhalation is the second major pathway of exposure, especially for workers in lead-related occupations. Almost all inhaled lead is absorbed into the body, the rate is 20–70% for ingested lead; children absorb more than adults.
Dermal exposure may be significant for a narrow category of people working with organic lead compounds, but is of little concern for general population. The rate of skin absorption is also low for inorganic lead.
According to Agency for Toxic Substance and Disease Registry, a small amount of lead (1%) will store itself in bones and the rest will be excreted through urine and feces within a few weeks of exposure. Children have a harder time excreting lead. Only about 32% of lead will be excreted by a child.
, paints and ceramic products, caulking
, and pipe solder has been dramatically reduced in recent years because of health concerns. Ingestion of contaminated food and drinking water is the most common source of lead exposure in humans. Exposure can also occur via inadvertent ingestion of contaminated soil/dust or lead-based paint.
Pharmaceuticals also require laboratory testing for heavy metals such as lead. The component limit of lead (1.0 μg/g) is a test benchmark for pharmaceuticals, representing the maximum daily intake an individual should have. However, even at this low level, a prolonged intake can be hazardous to human beings.
Chemical element
A chemical element is a pure chemical substance consisting of one type of atom distinguished by its atomic number, which is the number of protons in its nucleus. Familiar examples of elements include carbon, oxygen, aluminum, iron, copper, gold, mercury, and lead.As of November 2011, 118 elements...
in the carbon group
Carbon group
The carbon group is a periodic table group consisting of carbon , silicon , germanium , tin , lead , and ununquadium ....
with the symbol Pb (from ) and atomic number
Atomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
82. Lead is a soft, malleable poor metal. It is also counted as one of the heavy metals. Metallic lead has a bluish-white color after being freshly cut, but it soon tarnishes to a dull grayish color when exposed to air. Lead has a shiny chrome-silver luster when it is melted into a liquid.
Lead is used in building construction, lead-acid batteries, bullet
Bullet
A bullet is a projectile propelled by a firearm, sling, or air gun. Bullets do not normally contain explosives, but damage the intended target by impact and penetration...
s and shot
Lead shot
Lead shot is a collective term for small balls of lead. These were the original projectiles for muskets and early rifles, but today lead shot is fired primarily from shotguns. It is also used for a variety of other purposes...
s, weights, as part of solder
Solder
Solder is a fusible metal alloy used to join together metal workpieces and having a melting point below that of the workpiece.Soft solder is what is most often thought of when solder or soldering are mentioned and it typically has a melting range of . It is commonly used in electronics and...
s, pewter
Pewter
Pewter is a malleable metal alloy, traditionally 85–99% tin, with the remainder consisting of copper, antimony, bismuth and lead. Copper and antimony act as hardeners while lead is common in the lower grades of pewter, which have a bluish tint. It has a low melting point, around 170–230 °C ,...
s, fusible alloy
Fusible alloy
A fusible alloy is a metal alloy capable of being easily fused, i.e. easily meltable, at relatively low temperatures. Fusible alloys are commonly, but not necessarily, eutectic alloys....
s and as a radiation shield. Lead has the highest atomic number
Atomic number
In chemistry and physics, the atomic number is the number of protons found in the nucleus of an atom and therefore identical to the charge number of the nucleus. It is conventionally represented by the symbol Z. The atomic number uniquely identifies a chemical element...
of all of the stable elements, although the next higher element, bismuth
Bismuth
Bismuth is a chemical element with symbol Bi and atomic number 83. Bismuth, a trivalent poor metal, chemically resembles arsenic and antimony. Elemental bismuth may occur naturally uncombined, although its sulfide and oxide form important commercial ores. The free element is 86% as dense as lead...
, has a half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
that is so long (much longer than the age of the universe) that it can be considered stable. Its four stable isotopes have 82 proton
Proton
The proton is a subatomic particle with the symbol or and a positive electric charge of 1 elementary charge. One or more protons are present in the nucleus of each atom, along with neutrons. The number of protons in each atom is its atomic number....
s, a magic number
Magic number (physics)
In nuclear physics, a magic number is a number of nucleons such that they are arranged into complete shells within the atomic nucleus...
in the nuclear shell model of atomic nuclei.
Lead, at certain exposure levels, is a poisonous substance to animals as well as for human beings. It damages the nervous system
Nervous system
The nervous system is an organ system containing a network of specialized cells called neurons that coordinate the actions of an animal and transmit signals between different parts of its body. In most animals the nervous system consists of two parts, central and peripheral. The central nervous...
and causes brain
Brain
The brain is the center of the nervous system in all vertebrate and most invertebrate animals—only a few primitive invertebrates such as sponges, jellyfish, sea squirts and starfishes do not have one. It is located in the head, usually close to primary sensory apparatus such as vision, hearing,...
disorders. Excessive lead also causes blood disorders in mammals. Like the element mercury
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver or hydrargyrum...
, another heavy metal, lead is a neurotoxin
Neurotoxin
A neurotoxin is a toxin that acts specifically on nerve cells , usually by interacting with membrane proteins such as ion channels. Some sources are more general, and define the effect of neurotoxins as occurring at nerve tissue...
that accumulates both in soft tissues and the bones. Lead poisoning
Lead poisoning
Lead poisoning is a medical condition caused by increased levels of the heavy metal lead in the body. Lead interferes with a variety of body processes and is toxic to many organs and tissues including the heart, bones, intestines, kidneys, and reproductive and nervous systems...
has been documented from ancient Rome
Ancient Rome
Ancient Rome was a thriving civilization that grew on the Italian Peninsula as early as the 8th century BC. Located along the Mediterranean Sea and centered on the city of Rome, it expanded to one of the largest empires in the ancient world....
, ancient Greece
Ancient Greece
Ancient Greece is a civilization belonging to a period of Greek history that lasted from the Archaic period of the 8th to 6th centuries BC to the end of antiquity. Immediately following this period was the beginning of the Early Middle Ages and the Byzantine era. Included in Ancient Greece is the...
, and ancient China.
Characteristics
Lead is bright and silvery when freshly cut but the surface rapidly tarnishes in air to produce the commonly observed dull luster normally associated with lead. It is a denseDensity
The mass density or density of a material is defined as its mass per unit volume. The symbol most often used for density is ρ . In some cases , density is also defined as its weight per unit volume; although, this quantity is more properly called specific weight...
, ductile, very soft, highly malleable, bluish-white metal that has poor electrical conductivity when compared to most other metals. This metal is highly resistant to corrosion
Corrosion
Corrosion is the disintegration of an engineered material into its constituent atoms due to chemical reactions with its surroundings. In the most common use of the word, this means electrochemical oxidation of metals in reaction with an oxidant such as oxygen...
, and because of this property, it is used to contain corrosive liquids (for example, sulfuric acid
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
). Because lead is very malleable and resistant to corrosion it is extensively used in building construction – for example in the external coverings of roofing joints.
Metallic lead can be toughened by addition of small amounts of antimony
Antimony
Antimony is a toxic chemical element with the symbol Sb and an atomic number of 51. A lustrous grey metalloid, it is found in nature mainly as the sulfide mineral stibnite...
, or a small number of other metals such as calcium
Calcium
Calcium is the chemical element with the symbol Ca and atomic number 20. It has an atomic mass of 40.078 amu. Calcium is a soft gray alkaline earth metal, and is the fifth-most-abundant element by mass in the Earth's crust...
. All isotope
Isotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
s of lead, except for lead-204, can be found in the end products of the radioactive decay
Radioactive decay
Radioactive decay is the process by which an atomic nucleus of an unstable atom loses energy by emitting ionizing particles . The emission is spontaneous, in that the atom decays without any physical interaction with another particle from outside the atom...
of the even heavier elements, uranium
Uranium
Uranium is a silvery-white metallic chemical element in the actinide series of the periodic table, with atomic number 92. It is assigned the chemical symbol U. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons...
and thorium
Thorium
Thorium is a natural radioactive chemical element with the symbol Th and atomic number 90. It was discovered in 1828 and named after Thor, the Norse god of thunder....
.
Powdered lead burns with a bluish-white flame. As with many metals, finely divided powdered lead exhibits pyrophoricity
Pyrophoricity
A pyrophoric substance is a substance that will ignite spontaneously in air. Examples are iron sulfide and many reactive metals including uranium, when powdered or sliced thin. Pyrophoric materials are often water-reactive as well and will ignite when they contact water or humid air...
. Toxic fumes are released when lead is burned.
Isotopes
Lead can be found or produced in many isotopeIsotope
Isotopes are variants of atoms of a particular chemical element, which have differing numbers of neutrons. Atoms of a particular element by definition must contain the same number of protons but may have a distinct number of neutrons which differs from atom to atom, without changing the designation...
s, with three of them being stable. The four natural isotopes of lead are 204Pb, 206Pb, 207Pb, and 208Pb with the slightly radioactive 204Pb regarded as completely primordial lead, and the stable isotopes 206, 207, 208 being formed probably from the radioactive decay
Radioactive decay
Radioactive decay is the process by which an atomic nucleus of an unstable atom loses energy by emitting ionizing particles . The emission is spontaneous, in that the atom decays without any physical interaction with another particle from outside the atom...
of two isotopes of uranium (U-235
Uranium-235
- References :* .* DOE Fundamentals handbook: Nuclear Physics and Reactor theory , .* A piece of U-235 the size of a grain of rice can produce energy equal to that contained in three tons of coal or fourteen barrels of oil. -External links:* * * one of the earliest articles on U-235 for the...
and U-238
Uranium-238
Uranium-238 is the most common isotope of uranium found in nature. It is not fissile, but is a fertile material: it can capture a slow neutron and after two beta decays become fissile plutonium-239...
) and one isotope of thorium
Thorium
Thorium is a natural radioactive chemical element with the symbol Th and atomic number 90. It was discovered in 1828 and named after Thor, the Norse god of thunder....
(Th 232).
The one common radiogenic isotope of lead, 202Pb,it has a half-life
Half-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of about 53,000 years.
Chemistry
Various oxidized forms of lead are easily reduced to the metal. An example is heating PbO with mild organic reducing agents such as glucose. A mixture of the oxide and the sulfide heated together will also form the metal.- 2 PbO + PbS → 3 Pb + SO2
Metallic lead is attacked (oxidized) only superficially by air, forming a thin layer of lead oxide that protects it from further oxidation. The metal is not attacked by sulfuric
Sulfuric acid
Sulfuric acid is a strong mineral acid with the molecular formula . Its historical name is oil of vitriol. Pure sulfuric acid is a highly corrosive, colorless, viscous liquid. The salts of sulfuric acid are called sulfates...
or hydrochloric
Hydrochloric acid
Hydrochloric acid is a solution of hydrogen chloride in water, that is a highly corrosive, strong mineral acid with many industrial uses. It is found naturally in gastric acid....
acids. It dissolves in nitric acid
Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
with the evolution of nitric oxide
Nitric oxide
Nitric oxide, also known as nitrogen monoxide, is a diatomic molecule with chemical formula NO. It is a free radical and is an important intermediate in the chemical industry...
gas to form dissolved Pb(NO3)2
Lead(II) nitrate
Lead nitrate is an inorganic compound with the chemical formula Pb2. It commonly occurs as a colourless crystal or white powder and, unlike most other lead salts, is soluble in water....
.
- 3 Pb + 8 H+ + 8 → 3 Pb2+ + 6 + 2 NO + 4 H2O
When heated with nitrate
Nitrate
The nitrate ion is a polyatomic ion with the molecular formula NO and a molecular mass of 62.0049 g/mol. It is the conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically-bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a...
s of alkali metals, metallic lead oxidizes to form PbO
Lead(II) oxide
Lead oxide is the inorganic compound with the formula PbO. Lead oxide occurs in two polymorphs, red, having a tetragonal crystal structure and yellow, having an orthorhombic crystal structure...
(also known as litharge
Litharge
Litharge is one of the natural mineral forms of lead oxide, PbO. Litharge is a secondary mineral which forms from the oxidation of galena ores. It forms as coatings and encrustations with internal tetragonal crystal structure. It is dimorphous with the orthorhombic form massicot...
), leaving the corresponding alkali nitrite
Nitrite
The nitrite ion has the chemical formula NO2−. The anion is symmetric with equal N-O bond lengths and a O-N-O bond angle of ca. 120°. On protonation the unstable weak acid nitrous acid is produced. Nitrite can be oxidised or reduced, with product somewhat dependent on the oxidizing/reducing agent...
. PbO is representative of lead's +2 oxidation state. It is soluble in nitric
Nitric acid
Nitric acid , also known as aqua fortis and spirit of nitre, is a highly corrosive and toxic strong acid.Colorless when pure, older samples tend to acquire a yellow cast due to the accumulation of oxides of nitrogen. If the solution contains more than 86% nitric acid, it is referred to as fuming...
and acetic
Acetic acid
Acetic acid is an organic compound with the chemical formula CH3CO2H . It is a colourless liquid that when undiluted is also called glacial acetic acid. Acetic acid is the main component of vinegar , and has a distinctive sour taste and pungent smell...
acids, from which solutions it is possible to precipitate halide
Halide
A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides...
, sulfate, chromate, carbonate
Lead carbonate
Lead carbonate is the chemical compound PbCO3. It is prepared industrially from lead acetate and carbon dioxide.It occurs naturally as the mineral cerussite.-Basic lead carbonates:...
(PbCO3), and basic carbonate ( salts of lead. The sulfide
Lead sulfide
Lead sulfide is an ionic compound of lead and sulfur, having two possible proportions:*Lead sulfide, the ionic compound containing lead in the +2 oxidation state*Lead sulfide, the ionic compound containing lead in the +4 oxidation state...
can also be precipitated from acetate
Lead acetate
Lead acetate can refer to:* Lead acetate , Pb4* Lead acetate , Pb2...
solutions. These salts are all poorly soluble in water. Among the halides, the iodide is less soluble than the bromide, which, in turn, is less soluble than the chloride.
Lead(II) oxide is also soluble in alkali
Alkali metal
The alkali metals are a series of chemical elements in the periodic table. In the modern IUPAC nomenclature, the alkali metals comprise the group 1 elements, along with hydrogen. The alkali metals are lithium , sodium , potassium , rubidium , caesium , and francium...
hydroxide
Hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and a hydrogen atom held together by a covalent bond, and carrying a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, as a ligand, a nucleophile, and a...
solutions to form the corresponding plumbite
Plumbite
In chemistry, a plumbite is a salt having one of several lead-containing oxoanions in which lead is in the oxidation state +2. The term plumbite may also refer to the oxoanion itself...
salt.
- PbO + 2 OH− + H2O →
Chlorination
Chlorination
Chlorination is the process of adding the element chlorine to water as a method of water purification to make it fit for human consumption as drinking water...
of plumbite solutions causes the formation of lead's +4 oxidation state.
- + Cl2 → PbO2 + 2 Cl− + 2 H2O
Lead dioxide is representative of the +4 oxidation state, and is a powerful oxidizing agent
Oxidizing agent
An oxidizing agent can be defined as a substance that removes electrons from another reactant in a redox chemical reaction...
. The chloride of this oxidation state is formed only with difficulty and decomposes readily into lead(II) chloride and chlorine gas. The bromide and iodide of lead(IV) are not known to exist. Lead dioxide dissolves in alkali hydroxide solutions to form the corresponding plumbate
Plumbate
In chemistry, a plumbate is a salt having one of the several lead-containing oxoanions. Although the term plumbate can refer either to plumbate or plumbate, it traditionally refers specifically to plumbate, whereas plumbate is referred to as plumbite.Plumbates are formed by the reaction of lead...
s.
- PbO2 + 2 OH− + 2 H2O →
Lead also has an oxide with mixed +2 and +4 oxidation states, red lead , also known as minium.
Lead readily forms an equimolar alloy with sodium
Sodium
Sodium is a chemical element with the symbol Na and atomic number 11. It is a soft, silvery-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. It is an abundant element that exists in numerous minerals, most commonly as sodium chloride...
metal that reacts with alkyl halides to form organometallic compounds of lead such as tetraethyllead.
Chloride complexes

Chloride
The chloride ion is formed when the element chlorine, a halogen, picks up one electron to form an anion Cl−. The salts of hydrochloric acid HCl contain chloride ions and can also be called chlorides. The chloride ion, and its salts such as sodium chloride, are very soluble in water...
, the formation of which alters the corrosion chemistry of the lead. This will tend to limit the solubility of lead in saline
Salinity
Salinity is the saltiness or dissolved salt content of a body of water. It is a general term used to describe the levels of different salts such as sodium chloride, magnesium and calcium sulfates, and bicarbonates...
media.
| Pb2+ + Cl− → PbCl+ | K1 = 12.59 |
| PbCl+ + Cl− → PbCl2 | K2 = 14.45 |
| PbCl2 + Cl− → PbCl3− | K3 = 3.98 ×10−1 |
| PbCl3− + Cl− → PbCl42− | K4 = 8.92 × 10−2 |
Phase diagrams of solubilities
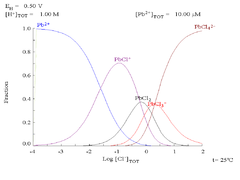
Lead(II) sulfate is poorly soluble, as can be seen in the following diagram showing addition of SO42− to a solution containing 0.1 M of Pb2+. The pH of the solution is 4.5, as above that, Pb2+ concentration can never reach 0.1 M due to the formation of Pb(OH)2. Observe that Pb2+ solubility drops 10,000 fold as SO42− reaches 0.1 M.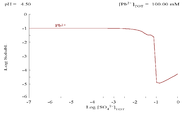 |
 |
| Plot showing aqueous concentration of dissolved Pb2+ as a function of SO42− | Diagram for lead in sulfate media |
The addition of chloride can lower the solubility of lead, though in chloride-rich media (such as aqua regia
Aqua regia
Aqua regia or aqua regis is a highly corrosive mixture of acids, fuming yellow or red solution, also called nitro-hydrochloric acid. The mixture is formed by freshly mixing concentrated nitric acid and hydrochloric acid, usually in a volume ratio of 1:3, respectively...
) the lead can become soluble again as anionic chloro-complexes.
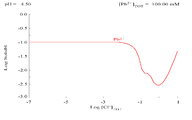 |
 |
| Diagram showing the solubility of lead in chloride media. The lead concentrations are plotted as a function of the total chloride present. | Pourbaix diagram Pourbaix diagram In chemistry, a Pourbaix diagram, also known as a potential/pH diagram, maps out possible stable phases of an aqueous electrochemical system. Predominant ion boundaries are represented by lines. As such a Pourbaix diagram can be read much like a standard phase diagram with a different set of axes... for lead in chloride (0.1 M) media |
History
Lead has been commonly used for thousands of years because it is widespread, easy to extract and easy to work with. It is highly malleable and ductile as well as easy to smeltSmelting
Smelting is a form of extractive metallurgy; its main use is to produce a metal from its ore. This includes iron extraction from iron ore, and copper extraction and other base metals from their ores...
. Metallic lead beads dating back to 6400 BCE have been found in Çatalhöyük
Çatalhöyük
Çatalhöyük was a very large Neolithic and Chalcolithic settlement in southern Anatolia, which existed from approximately 7500 BCE to 5700 BCE...
in modern-day Turkey. In the early Bronze Age
Bronze Age
The Bronze Age is a period characterized by the use of copper and its alloy bronze as the chief hard materials in the manufacture of some implements and weapons. Chronologically, it stands between the Stone Age and Iron Age...
, lead was used with antimony
Antimony
Antimony is a toxic chemical element with the symbol Sb and an atomic number of 51. A lustrous grey metalloid, it is found in nature mainly as the sulfide mineral stibnite...
and arsenic
Arsenic
Arsenic is a chemical element with the symbol As, atomic number 33 and relative atomic mass 74.92. Arsenic occurs in many minerals, usually in conjunction with sulfur and metals, and also as a pure elemental crystal. It was first documented by Albertus Magnus in 1250.Arsenic is a metalloid...
.
The largest preindustrial
Industrial Revolution
The Industrial Revolution was a period from the 18th to the 19th century where major changes in agriculture, manufacturing, mining, transportation, and technology had a profound effect on the social, economic and cultural conditions of the times...
producer of lead was the Roman economy
Roman economy
The history of the Roman economy covers the period of the Roman Republic and the Roman Empire.Recent research has led to a positive reevaluation of the size and sophistication of the Roman economy within the constraints generally imposed on agricultural societies in the preindustrial age.- Gross...
, with an estimated output per annum of 80,000 t, which was typically won as a by-product of extensive silver smelting. Roman mining activities
Roman metallurgy
Metals and metal working had been known to the people of modern Italy since the Bronze Age. By 86 BC, Rome had already expanded to control an immense expanse of the Mediterranean...
occurred in Central Europe
Central Europe
Central Europe or alternatively Middle Europe is a region of the European continent lying between the variously defined areas of Eastern and Western Europe...
, Roman Britain
Roman Britain
Roman Britain was the part of the island of Great Britain controlled by the Roman Empire from AD 43 until ca. AD 410.The Romans referred to the imperial province as Britannia, which eventually comprised all of the island of Great Britain south of the fluid frontier with Caledonia...
, the Balkans
Balkans
The Balkans is a geopolitical and cultural region of southeastern Europe...
, Greece
Greece
Greece , officially the Hellenic Republic , and historically Hellas or the Republic of Greece in English, is a country in southeastern Europe....
, Asia Minor
Asia Minor
Asia Minor is a geographical location at the westernmost protrusion of Asia, also called Anatolia, and corresponds to the western two thirds of the Asian part of Turkey...
; Hispania
Hispania
Another theory holds that the name derives from Ezpanna, the Basque word for "border" or "edge", thus meaning the farthest area or place. Isidore of Sevilla considered Hispania derived from Hispalis....
alone accounted for 40% of world production.
Roman lead pipes often bore the insignia of Roman emperors (see Roman lead pipe inscription
Roman lead pipe inscription
A Roman lead pipe inscription is a Latin inscription on a Roman water pipe made of lead which provides brief information on its manufacturer and owner, often the reigning emperor himself as the supreme authority...
s). Lead plumbing in the Latin West may have been continued beyond the age of Theoderic the Great into the medieval period. Many Roman "pigs" (ingots) of lead figure in Derbyshire lead mining history
Derbyshire lead mining history
This article details some of the history of lead mining in Derbyshire, England.- Background :On one of the walls in Wirksworth church is a crude stone carving, found nearby at Bonsall and placed in the church in the 1870s. Probably executed in Anglo-Saxon times, it shows a man carrying a kibble or...
and in the history of the industry in other English centers. The Romans also used lead in molten form to secure iron pins that held together large limestone
Limestone
Limestone is a sedimentary rock composed largely of the minerals calcite and aragonite, which are different crystal forms of calcium carbonate . Many limestones are composed from skeletal fragments of marine organisms such as coral or foraminifera....
blocks in certain monumental buildings. In alchemy
Alchemy
Alchemy is an influential philosophical tradition whose early practitioners’ claims to profound powers were known from antiquity. The defining objectives of alchemy are varied; these include the creation of the fabled philosopher's stone possessing powers including the capability of turning base...
, lead was thought to be the oldest metal and was associated with the planet Saturn
Saturn
Saturn is the sixth planet from the Sun and the second largest planet in the Solar System, after Jupiter. Saturn is named after the Roman god Saturn, equated to the Greek Cronus , the Babylonian Ninurta and the Hindu Shani. Saturn's astronomical symbol represents the Roman god's sickle.Saturn,...
. Alchemists accordingly used Saturn's symbol (the scythe) to refer to lead.
Lead's symbol Pb is an abbreviation of its Latin
Latin
Latin is an Italic language originally spoken in Latium and Ancient Rome. It, along with most European languages, is a descendant of the ancient Proto-Indo-European language. Although it is considered a dead language, a number of scholars and members of the Christian clergy speak it fluently, and...
name plumbum for soft metals; originally it was plumbum nigrum (literally, "black plumbum"), where plumbum candidum (literally, "bright plumbum") was tin
Tin
Tin is a chemical element with the symbol Sn and atomic number 50. It is a main group metal in group 14 of the periodic table. Tin shows chemical similarity to both neighboring group 14 elements, germanium and lead and has two possible oxidation states, +2 and the slightly more stable +4...
. The English words "plumbing
Plumbing
Plumbing is the system of pipes and drains installed in a building for the distribution of potable drinking water and the removal of waterborne wastes, and the skilled trade of working with pipes, tubing and plumbing fixtures in such systems. A plumber is someone who installs or repairs piping...
", "plumber
Plumber
A plumber is a tradesperson who specializes in installing and maintaining systems used for potable water, sewage, and drainage in plumbing systems. The term dates from ancient times, and is related to the Latin word for lead, "plumbum." A person engaged in fixing metaphorical "leaks" may also be...
", "plumb", and "plumb-bob
Plumb-bob
A plumb-bob or a plummet is a weight, usually with a pointed tip on the bottom, that is suspended from a string and used as a vertical reference line, or plumb-line....
" also derive from this Latin root.
Occurrence
Metallic lead does occur in nature, but it is rare. Lead is usually found in oreOre
An ore is a type of rock that contains minerals with important elements including metals. The ores are extracted through mining; these are then refined to extract the valuable element....
with zinc
Zinc
Zinc , or spelter , is a metallic chemical element; it has the symbol Zn and atomic number 30. It is the first element in group 12 of the periodic table. Zinc is, in some respects, chemically similar to magnesium, because its ion is of similar size and its only common oxidation state is +2...
, silver
Silver
Silver is a metallic chemical element with the chemical symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal...
and (most abundantly) copper
Copper
Copper is a chemical element with the symbol Cu and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is soft and malleable; an exposed surface has a reddish-orange tarnish...
, and is extracted together with these metals. The main lead mineral
Mineral
A mineral is a naturally occurring solid chemical substance formed through biogeochemical processes, having characteristic chemical composition, highly ordered atomic structure, and specific physical properties. By comparison, a rock is an aggregate of minerals and/or mineraloids and does not...
is galena
Galena
Galena is the natural mineral form of lead sulfide. It is the most important lead ore mineral.Galena is one of the most abundant and widely distributed sulfide minerals. It crystallizes in the cubic crystal system often showing octahedral forms...
(PbS), which contains 86.6 % lead by weight. Other common varieties are cerussite
Cerussite
Cerussite is a mineral consisting of lead carbonate , and an important ore of lead. The name is from the Latin cerussa, white lead. Cerussa nativa was mentioned by Conrad Gessner in 1565, and in 1832 F. S. Beudant applied the name cruse to the mineral, whilst the present form, cerussite, is due to...
(PbCO3) and anglesite
Anglesite
Anglesite is a lead sulfate mineral with the chemical formula PbSO4. It occurs as an oxidation product of primary lead sulfide ore, galena. Anglesite occurs as prismatic orthorhombic crystals and earthy masses, and is isomorphous with barite and celestine. It contains 74% of lead by mass and...
(PbSO4).
Ore processing
Most ores contain less than 10% lead, and ores containing as little as 3% lead can be economically exploited. Ores are crushed and concentrated by froth flotationFroth flotation
Froth flotation is a process for selectively separating hydrophobic materials from hydrophilic. This is used in several processing industries...
typically to 70% or more. Sulfide
Sulfide
A sulfide is an anion of sulfur in its lowest oxidation state of 2-. Sulfide is also a slightly archaic term for thioethers, a common type of organosulfur compound that are well known for their bad odors.- Properties :...
ores are roasted
Roasting (metallurgy)
Roasting is a step in the processing of certain ores. More specifically, roasting is a metallurgical process involving gas–solid reactions at elevated temperatures with the goal of purifying the metal component. Often before roasting, the ore has already been partially purified, e.g. by froth...
, producing primarily lead oxide and a mixture of sulfates and silicates of lead and other metals contained in the ore.
Lead oxide from the roasting process is reduced in a coke-fired blast furnace
Blast furnace
A blast furnace is a type of metallurgical furnace used for smelting to produce industrial metals, generally iron.In a blast furnace, fuel and ore and flux are continuously supplied through the top of the furnace, while air is blown into the bottom of the chamber, so that the chemical reactions...
. This converts most of the lead to its metallic form. Three additional layers separate in the process and float to the top of the metallic lead. These are slag
Slag
Slag is a partially vitreous by-product of smelting ore to separate the metal fraction from the unwanted fraction. It can usually be considered to be a mixture of metal oxides and silicon dioxide. However, slags can contain metal sulfides and metal atoms in the elemental form...
(silicates containing 1.5% lead), matte
Matte (metallurgy)
Matte is a term used in the field of pyrometallurgy given to the molten metal sulfide phases typically formed during smelting of copper, nickel, and other base metals. Typically, a matte is the phase in which the principal metal being extracted is recovered prior to a final reduction process to...
(sulfides containing 15% lead), and speiss
Speiss
Speiss is a molten phase consisting primarily of iron arsenide that is commonly encountered in lead smelting operations....
(arsenides of iron and copper). These wastes contain concentrations of copper, zinc, cadmium, and bismuth that can be recovered economically, as can their content of unreduced lead.
Metallic lead that results from the roasting and blast furnace processes still contains significant contaminants of arsenic, antimony, bismuth, zinc, copper, silver, and gold. The melt is treated in a reverberatory furnace
Reverberatory furnace
A reverberatory furnace is a metallurgical or process furnace that isolates the material being processed from contact with the fuel, but not from contact with combustion gases...
with air, steam, and sulfur, which oxidizes the contaminants except silver, gold, and bismuth. The oxidized contaminants are removed by dross
Dross
Dross is a mass of solid impurities floating on a molten metal. It appears usually on the melting of low-melting-point metals or alloys such as tin, lead, zinc or aluminium, or by oxidation of the metal. It can also consist of impurities such as paint leftovers...
ing, where they float to the top and are skimmed off.
Most lead ores contain significant concentrations of silver
Silver
Silver is a metallic chemical element with the chemical symbol Ag and atomic number 47. A soft, white, lustrous transition metal, it has the highest electrical conductivity of any element and the highest thermal conductivity of any metal...
, resulting in the smelted metal also containing silver as a contaminant. Metallic silver as well as gold is removed and recovered economically by means of the Parkes process
Parkes process
The Parkes process is a pyrometallurgical industrial process for removing silver from lead, during the production of bullion. It is an example of liquid-liquid extraction....
.
Desilvered lead is freed of bismuth
Bismuth
Bismuth is a chemical element with symbol Bi and atomic number 83. Bismuth, a trivalent poor metal, chemically resembles arsenic and antimony. Elemental bismuth may occur naturally uncombined, although its sulfide and oxide form important commercial ores. The free element is 86% as dense as lead...
according to the Betterton-Kroll process
Betterton-Kroll process
The Betterton-Kroll process is an industrial process for removing bismuth from lead.Calcium and magnesium are added to a molten lead-bismuth bath. The resulting bismuth compounds have higher melting points and lower densities than the lead, and can be removed as dross. The compounds are treated...
by treating it with metallic calcium and magnesium, which forms a bismuth dross that can be skimmed off.
Very pure lead can be obtained by processing smelted lead electrolytically by means of the Betts process
Betts electrolytic process
The Betts electrolytic process is an industrial process for separating lead and bismuth. It is named for its inventor Anson Gardner Betts.-Process description for lead:...
. The process uses anodes of impure lead and cathodes of pure lead in an electrolyte of silica fluoride.
Production and recycling
Production and consumption of lead is increasing worldwide. Total annual production is about 8 million tonnes; about half is produced from recycled scrap. The top lead producing countries, as of 2008, are Australia, China, USA, Peru, Canada, Mexico, Sweden, Morocco, South Africa and North Korea. Australia, China and the United States account for more than half of primary production., 9.6 million tonnes of lead were produced, of which 4.1 million tonnes came from mining.
At current use rates, the supply of lead is estimated to run out in 42 years. Environmental analyst Lester Brown has suggested lead could run out within 18 years based on an extrapolation of 2% growth per year. This may need to be reviewed to take account of renewed interest in recycling
Recycling
Recycling is processing used materials into new products to prevent waste of potentially useful materials, reduce the consumption of fresh raw materials, reduce energy usage, reduce air pollution and water pollution by reducing the need for "conventional" waste disposal, and lower greenhouse...
, and rapid progress in fuel cell
Fuel cell
A fuel cell is a device that converts the chemical energy from a fuel into electricity through a chemical reaction with oxygen or another oxidizing agent. Hydrogen is the most common fuel, but hydrocarbons such as natural gas and alcohols like methanol are sometimes used...
technology. According to the International Resource Panel
International Resource Panel
The International Resource Panel is a scientific panel of experts that aims to help nations use natural resources sustainably without compromising economic growth and human needs...
's Metal Stocks in Society report
Metal Stocks in Society report
The report Metal Stocks in Society: Scientific Synthesis was the first of six scientific assessments on global metals to be published by the International Resource Panel of the United Nations Environment Programme...
, the global per capita stock of lead in use in society is 8 kg. Much of this is in more-developed countries (20–150 kg per capita) rather than less-developed countries (1–4 kg per capita).
Applications
Due to its half-lifeHalf-life
Half-life, abbreviated t½, is the period of time it takes for the amount of a substance undergoing decay to decrease by half. The name was originally used to describe a characteristic of unstable atoms , but it may apply to any quantity which follows a set-rate decay.The original term, dating to...
of 22.2 years, the radioactive isotope 210Pb
Isotopes of lead
Lead has four stable isotopes: 204Pb, 206Pb, 207Pb, 208Pb. Lead-204 is entirely a primordial nuclide and is not a radiogenic nuclide. The three isotopes lead-206, lead-207, and lead-208 represent the ends of three decay chains called the uranium series , the actinium series, and the thorium...
is used for dating material from marine sediment
Sediment
Sediment is naturally occurring material that is broken down by processes of weathering and erosion, and is subsequently transported by the action of fluids such as wind, water, or ice, and/or by the force of gravity acting on the particle itself....
cores by radiometric
Radiometric dating
Radiometric dating is a technique used to date materials such as rocks, usually based on a comparison between the observed abundance of a naturally occurring radioactive isotope and its decay products, using known decay rates...
methods.
Elemental lead

Sailing ballast
Ballast is used in sailboats to provide moment to resist the lateral forces on the sail. Insufficiently ballasted boats will tend to tip, or heel, excessively in high winds. Too much heel may result in the boat capsizing. If a sailing vessel should need to voyage without cargo then ballast of...
keel of sailboats. Its high density allows it to counterbalance the heeling effect of wind on the sails while at the same time occupying a small volume and thus offering the least underwater resistance. For the same reason it is used in scuba diving
Scuba diving
Scuba diving is a form of underwater diving in which a diver uses a scuba set to breathe underwater....
weight belts
Diving weighting system
Divers wear weighting systems, weight belts or weights, generally made of lead, to counteract the buoyancy of other diving equipment, such as diving suits and aluminium diving cylinders...
to counteract the diver's natural buoyancy and that of his equipment. It does not have the weight-to-volume ratio of many heavy metals, but its low cost increases its use in these and other applications.


Hot metal typesetting
In printing and typography, hot metal typesetting refers to 19th-century technologies for typesetting text in letterpress printing. This method injects molten type metal into a mold that has the shape of one or more glyphs...
uses a lead based alloy to produce the types for printing directly before printing.
Its corrosion resistance makes it suitable for outdoor applications when in contact with water.
More than half of the worldwide lead production (at least 1.15 million metric tons) is used for automobiles, mostly as electrodes in the lead–acid battery, used extensively as a car battery
Car battery
An automotive battery is a type of rechargeable battery that supplies electric energy to an automobile. Usually this refers to an SLI battery to power the starter motor, the lights, and the ignition system of a vehicle’s engine...
.
Cathode
Cathode
A cathode is an electrode through which electric current flows out of a polarized electrical device. Mnemonic: CCD .Cathode polarity is not always negative...
(reduction
Redox
Redox reactions describe all chemical reactions in which atoms have their oxidation state changed....
)
- PbO2 + 4 H+ + SO42– + 2e– → PbSO4 + 2 H2O
Anode
Anode
An anode is an electrode through which electric current flows into a polarized electrical device. Mnemonic: ACID ....
(oxidation)
- Pb + SO42– → PbSO4 + 2e–
Lead is used as electrodes in the process of electrolysis
Electrolysis
In chemistry and manufacturing, electrolysis is a method of using a direct electric current to drive an otherwise non-spontaneous chemical reaction...
. Lead is used in solder
Solder
Solder is a fusible metal alloy used to join together metal workpieces and having a melting point below that of the workpiece.Soft solder is what is most often thought of when solder or soldering are mentioned and it typically has a melting range of . It is commonly used in electronics and...
for electronics, although this usage is being phased out by some countries to reduce the amount of environmentally hazardous waste. Lead is used in high voltage power cables as sheathing material to prevent water diffusion into insulation.
Lead is one of three metals used in the Oddy test
Oddy test
The Oddy test is a procedure created at the British Museum by conservation scientist Andrew Oddy in 1973, in order to test materials for safety in and around art objects....
for museum materials, helping detect organic acids, aldehydes, and acidic gases.
Lead is used as shielding
Lead shielding
Lead shielding refers to the use of lead as a form of radiation protection to shield people or objects from radiation. Lead can effectively attenuate certain kinds of radiation because of its high density and high atomic number; principally, it is effective at stopping alpha rays, gamma rays, and...
from radiation
Ionizing radiation
Ionizing radiation is radiation composed of particles that individually have sufficient energy to remove an electron from an atom or molecule. This ionization produces free radicals, which are atoms or molecules containing unpaired electrons...
(e.g., in X-ray
X-ray
X-radiation is a form of electromagnetic radiation. X-rays have a wavelength in the range of 0.01 to 10 nanometers, corresponding to frequencies in the range 30 petahertz to 30 exahertz and energies in the range 120 eV to 120 keV. They are shorter in wavelength than UV rays and longer than gamma...
rooms). Molten lead is used as a coolant
Coolant
A coolant is a fluid which flows through a device to prevent its overheating, transferring the heat produced by the device to other devices that use or dissipate it. An ideal coolant has high thermal capacity, low viscosity, is low-cost, non-toxic, and chemically inert, neither causing nor...
(e.g., for lead cooled fast reactor
Lead cooled fast reactor
The lead-cooled fast reactor is a nuclear power Generation IV reactor that features a fast neutron spectrum, molten lead or lead-bismuth eutectic coolant. Options include a range of plant ratings, including a number of 50 to 150 MWe units featuring long-life, pre-manufactured cores...
s).
Lead is added to brass
Brass
Brass is an alloy of copper and zinc; the proportions of zinc and copper can be varied to create a range of brasses with varying properties.In comparison, bronze is principally an alloy of copper and tin...
to reduce machine tool
Machine tool
A machine tool is a machine, typically powered other than by human muscle , used to make manufactured parts in various ways that include cutting or certain other kinds of deformation...
wear.
Lead, in the form of strips, or tape, is used for the customization of tennis rackets. Tennis rackets of the past sometimes had lead added to them by the manufacturer to increase weight.
Lead is used to form glazing bars for stained glass
Stained glass
The term stained glass can refer to coloured glass as a material or to works produced from it. Throughout its thousand-year history, the term has been applied almost exclusively to the windows of churches and other significant buildings...
or other multi-lit windows. The practice has become less common, not for danger but for stylistic reasons.
Lead, or sheet-lead, is used as a sound deadening layer in some areas in wall, floor and ceiling design in sound studios where levels of airborne and mechanically produced sound are targeted for reduction or virtual elimination.
Lead is the traditional base metal of organ pipe
Organ pipe
An organ pipe is a sound-producing element of the pipe organ that resonates at a specific pitch when pressurized air is driven through it. Each pipe is tuned to a specific note of the musical scale...
s, mixed with varying amounts of tin
Tin
Tin is a chemical element with the symbol Sn and atomic number 50. It is a main group metal in group 14 of the periodic table. Tin shows chemical similarity to both neighboring group 14 elements, germanium and lead and has two possible oxidation states, +2 and the slightly more stable +4...
to control the tone of the pipe.
Lead has many uses in the construction industry (e.g., lead sheets are used as architectural metals
Architectural metals
Architectural metals used in buildings and structures comprise several distinctive metallic materials. Metals serve a wide variety of uses in the built landscape, including structural features, such as nails and trusses, as well as decorative features, such as doorknobs and cladding. Some metals...
in roofing material, cladding, flashing, gutters and gutter joints, and on roof parapets). Detailed lead moldings are used as decorative motifs used to fix lead sheet. Lead is still widely used in statues and sculptures.
Lead is often used to balance
Tire balance
Tire balance, also referred to as tire unbalance or imbalance, describes the distribution of mass within an automobile tire or the wheel to which it is attached. When the tire rotates, asymmetries of mass cause the wheel to wobble, which can cause ride disturbances, usually vertical and lateral...
the wheels of a car; this use is being phased out in favor of other materials for environmental reasons.
Lead compounds
Lead compounds are used as a coloring element in ceramic glazeCeramic glaze
Glaze is a layer or coating of a vitreous substance which has been fired to fuse to a ceramic object to color, decorate, strengthen or waterproof it.-Use:...
s, notably in the colors red and yellow.
Lead is frequently used in polyvinyl chloride
Polyvinyl chloride
Polyvinyl chloride, commonly abbreviated PVC, is a thermoplastic polymer. It is a vinyl polymer constructed of repeating vinyl groups having one hydrogen replaced by chloride. Polyvinyl chloride is the third most widely produced plastic, after polyethylene and polypropylene. PVC is widely used in...
(PVC) plastic, which coats electrical cords.
Lead is used in some candles to treat the wick to ensure a longer, more even burn. Because of the dangers, European and North American manufacturers use more expensive alternatives such as zinc. Lead glass
Lead glass
Lead glass is a variety of glass in which lead replaces the calcium content of a typical potash glass. Lead glass contains typically 18–40 weight% lead oxide , while modern lead crystal, historically also known as flint glass due to the original silica source, contains a minimum of 24% PbO...
is composed of 12–28% lead oxide
Lead(II) oxide
Lead oxide is the inorganic compound with the formula PbO. Lead oxide occurs in two polymorphs, red, having a tetragonal crystal structure and yellow, having an orthorhombic crystal structure...
. It changes the optical characteristics of the glass and reduces the transmission of radiation.
Some artists using oil-based paints continue to use lead carbonate white, citing its properties in comparison with the alternatives. Tetra-ethyl lead is used as an anti-knock additive for aviation fuel in piston-driven aircraft. Lead-based semiconductors, such as lead telluride, lead selenide and lead antimonide are finding applications in photovoltaic (solar energy) cells and infrared
Infrared
Infrared light is electromagnetic radiation with a wavelength longer than that of visible light, measured from the nominal edge of visible red light at 0.74 micrometres , and extending conventionally to 300 µm...
detectors.
Former applications
Lead pigments were used in lead paintLead paint
Lead paint or lead-based paint is paint containing lead, a heavy metal, that is used as pigment, with lead chromate and lead carbonate being the most common. Lead is also added to paint to speed drying, increase durability, retain a fresh appearance, and resist moisture that causes corrosion...
for white as well as yellow
Chrome yellow
Chrome Yellow is a natural yellow pigment made of lead chromate . It was first extracted from the mineral crocoite by the French chemist Louis Vauquelin in 1809...
, orange, and red. Most uses have been discontinued due of the dangers of lead poisoning. Beginning April 22, 2010, US federal law requires that contractors performing renovation, repair, and painting projects that disturb more than six square feet of paint in homes, child care facilities, and schools built before 1978 must be certified and trained to follow specific work practices to prevent lead contamination. Lead chromate is still in industrial use. Lead carbonate (white) is the traditional pigment for the priming medium for oil painting, but it has been largely displaced by the zinc and titanium oxide pigments. It was also quickly replaced in water-based painting mediums. Lead carbonate white was used by the Japanese geisha
Geisha
, Geiko or Geigi are traditional, female Japanese entertainers whose skills include performing various Japanese arts such as classical music and dance.-Terms:...
and in the West for face-whitening make-up, which was detrimental to health.
Lead is the hot metal that was used in hot metal typesetting
Hot metal typesetting
In printing and typography, hot metal typesetting refers to 19th-century technologies for typesetting text in letterpress printing. This method injects molten type metal into a mold that has the shape of one or more glyphs...
. It was used for plumbing
Plumbing
Plumbing is the system of pipes and drains installed in a building for the distribution of potable drinking water and the removal of waterborne wastes, and the skilled trade of working with pipes, tubing and plumbing fixtures in such systems. A plumber is someone who installs or repairs piping...
as well as a preservative
Food preservation
Food preservation is the process of treating and handling food to stop or slow down spoilage and thus allow for longer storage....
for food and drink in Ancient Rome
Ancient Rome
Ancient Rome was a thriving civilization that grew on the Italian Peninsula as early as the 8th century BC. Located along the Mediterranean Sea and centered on the city of Rome, it expanded to one of the largest empires in the ancient world....
. Until the early 1970s, lead was used for joining cast iron water pipes and used as a material for small diameter water pipes.
Tetraethyllead was used in leaded fuels to reduce engine knocking
Engine knocking
Knocking in spark-ignition internal combustion engines occurs when combustion of the air/fuel mixture in the cylinder starts off correctly in response to ignition by the spark plug, but one or more pockets of air/fuel mixture explode outside the envelope of the normal combustion front.The...
, but this practice has been phased out across many countries of the world in efforts to reduce toxic pollution that affected humans and the environment.
Lead was used to make bullets for slings
Sling (weapon)
A sling is a projectile weapon typically used to throw a blunt projectile such as a stone or lead "sling-bullet". It is also known as the shepherd's sling....
. Lead was used for shotgun
Shotgun
A shotgun is a firearm that is usually designed to be fired from the shoulder, which uses the energy of a fixed shell to fire a number of small spherical pellets called shot, or a solid projectile called a slug...
pellets in the US until about 1992 when it was outlawed (for waterfowl
Waterfowl
Waterfowl are certain wildfowl of the order Anseriformes, especially members of the family Anatidae, which includes ducks, geese, and swans....
hunting only) and replaced by non-toxic shot, primarily steel pellets. In the Netherlands
Netherlands
The Netherlands is a constituent country of the Kingdom of the Netherlands, located mainly in North-West Europe and with several islands in the Caribbean. Mainland Netherlands borders the North Sea to the north and west, Belgium to the south, and Germany to the east, and shares maritime borders...
, the use of lead shot for hunting and sport shooting was banned in 1993, which caused a large drop in lead emission, from 230 ton in 1990 to 47.5 ton in 1995, two years after the ban.
Lead was a component of the paint used on children's toys – now restricted in the United States and across Europe (ROHS Directive). Lead was used in car body filler, which was used in many custom car
Custom car
A custom car is a passenger vehicle that has been modified in either of the following two ways. First, a custom car may be altered to improve its performance, often by altering or replacing the engine and transmission. Second, a custom car may be a personal "styling" statement, making the car look...
s in the 1940s–60s. Hence the term Leadsled. Lead is a superconductor at 7.2 K and IBM
IBM
International Business Machines Corporation or IBM is an American multinational technology and consulting corporation headquartered in Armonk, New York, United States. IBM manufactures and sells computer hardware and software, and it offers infrastructure, hosting and consulting services in areas...
tried to make a Josephson effect
Josephson effect
The Josephson effect is the phenomenon of supercurrent across two superconductors coupled by a weak link...
computer out of lead-alloy.
Lead was also used in pesticides before the 1950s, when fruit orchards were treated (ATSDR). A lead cylinder attached to a long line was used by sailors for the vital navigational task of determining water depth by heaving the lead at regular internals. A soft tallow insert at its base allowed the nature of the sea bed to be determined, further aiding position finding. Contrary to popular belief, pencil leads in wooden pencils have never been made from lead. The term comes from the Roman stylus, called the penicillus, which was made of lead without a wooden holder. When the pencil originated as a wrapped graphite writing tool, the particular type of graphite
Graphite
The mineral graphite is one of the allotropes of carbon. It was named by Abraham Gottlob Werner in 1789 from the Ancient Greek γράφω , "to draw/write", for its use in pencils, where it is commonly called lead . Unlike diamond , graphite is an electrical conductor, a semimetal...
being used was named plumbago (lit. act for lead, or lead mockup).
Health effects
Lead is a poisonous metal that can damage nervous connections (especially in young children) and cause blood and brain disorders. Lead poisoning typically results from ingestion of food or water contaminated with lead; but may also occur after accidental ingestion of contaminated soil, dust, or lead based paint. Long-term exposure to lead or its salts (especially soluble salts or the strong oxidant PbO2) can cause nephropathyNephropathy
Nephropathy refers to damage to or disease of the kidney. An older term for this is nephrosis.-Causes:Causes of nephropathy include administration of analgesics, xanthine oxidase deficiency, and long-term exposure to lead or its salts...
, and colic
Colic
Colic is a form of pain which starts and stops abruptly. Types include:*Baby colic, a condition, usually in infants, characterized by incessant crying*Renal colic, a pain in the flank, characteristic of kidney stones...
-like abdominal pains. The effects of lead are the same whether it enters the body through breathing or swallowing. Lead can affect almost every organ and system in the body. The main target for lead toxicity is the nervous system, both in adults and children. Long-term exposure of adults can result in decreased performance in some tests that measure functions of the nervous system. It may also cause weakness in fingers, wrists, or ankles. Lead exposure also causes small increases in blood pressure, particularly in middle-aged and older people and can cause anemia. Exposure to high lead levels can severely damage the brain and kidneys in adults or children and ultimately cause death. In pregnant women, high levels of exposure to lead may cause miscarriage. Chronic, high-level exposure have shown to reduce fertility in males. The antidote/treatment for lead poisoning consists of dimercaprol
Dimercaprol
Dimercaprol or British anti-Lewisite , is a compound developed by British biochemists at Oxford University during World War II. It was developed secretly as an antidote for lewisite, the now-obsolete arsenic-based chemical warfare agent. Today, it is used medically in treatment of arsenic,...
and succimer.
| NFPA 704 |
|---|
| Fire diamond for lead granules |
The concern about lead's role in cognitive deficits in children has brought about widespread reduction in its use (lead exposure has been linked to learning disabilities). Most cases of adult elevated blood lead levels are workplace-related. High blood levels are associated with delayed puberty in girls. Lead has been shown many times to permanently reduce the cognitive capacity of children at extremely low levels of exposure.
During the 20th century, the use of lead in paint pigment
Pigment
A pigment is a material that changes the color of reflected or transmitted light as the result of wavelength-selective absorption. This physical process differs from fluorescence, phosphorescence, and other forms of luminescence, in which a material emits light.Many materials selectively absorb...
s was sharply reduced because of the danger of lead poisoning, especially to children. By the mid-1980s, a significant shift in lead end-use patterns had taken place. Much of this shift was a result of the U.S. lead consumers' compliance with environmental regulations that significantly reduced or eliminated the use of lead in non-battery products, including gasoline
Gasoline
Gasoline , or petrol , is a toxic, translucent, petroleum-derived liquid that is primarily used as a fuel in internal combustion engines. It consists mostly of organic compounds obtained by the fractional distillation of petroleum, enhanced with a variety of additives. Some gasolines also contain...
, paints, solders, and water systems. Lead use is being further curtailed by the European Union's RoHS directive
Restriction of Hazardous Substances Directive
The Directive on the restriction of the use of certain hazardous substances in electrical and electronic equipment 2002/95/EC was adopted in February 2003 by the European Union. The RoHS directive took effect on 1 July 2006, and is required to be enforced and become law in each member state...
. Lead may still be found in harmful quantities in stoneware, vinyl (such as that used for tubing and the insulation of electrical cords), and brass manufactured in China. Between 2006 and 2007 many children's toys made in China were recalled, primarily due to lead in paint used to color the product.
Older houses may still contain substantial amounts of lead paint
Lead paint
Lead paint or lead-based paint is paint containing lead, a heavy metal, that is used as pigment, with lead chromate and lead carbonate being the most common. Lead is also added to paint to speed drying, increase durability, retain a fresh appearance, and resist moisture that causes corrosion...
. White lead paint has been withdrawn from sale in industrialized countries, but the yellow lead chromate is still in use; for example, Holland Colours Holcolan Yellow. Old paint should not be stripped by sanding, as this produces inhalable dust.
Lead salts used in pottery glazes have on occasion caused poisoning, when acidic drinks, such as fruit juices, have leached lead ions out of the glaze. It has been suggested that what was known as "Devon colic
Devon colic
Devon colic is an affliction caused by lead poisoning which was suffered by the people of Devon during parts of the 17th and 18th centuries....
" arose from the use of lead-lined presses to extract apple juice in the manufacture of cider
Cider
Cider or cyder is a fermented alcoholic beverage made from apple juice. Cider varies in alcohol content from 2% abv to 8.5% abv or more in traditional English ciders. In some regions, such as Germany and America, cider may be termed "apple wine"...
. Lead is considered to be particularly harmful for women's ability to reproduce. Lead(II) acetate
Lead(II) acetate
Lead acetate , also known as lead acetate, lead diacetate, plumbous acetate, sugar of lead, lead sugar, salt of Saturn, and Goulard's powder, is a white crystalline chemical compound with a sweetish taste. It is made by treating lead oxide with acetic acid. Like other lead compounds, it is toxic...
(also known as sugar of lead) was used by the Roman Empire
Roman Empire
The Roman Empire was the post-Republican period of the ancient Roman civilization, characterised by an autocratic form of government and large territorial holdings in Europe and around the Mediterranean....
as a sweetener for wine, and some consider this to be the cause of the dementia
Dementia
Dementia is a serious loss of cognitive ability in a previously unimpaired person, beyond what might be expected from normal aging...
that affected many of the Roman Emperors.
Lead as a soil contaminant is a widespread issue, since lead is present in natural deposits and may also enter soil through (leaded) gasoline leaks from underground storage tank
Underground storage tank
An Underground Storage Tank , in United States environmental law, is a tank and any underground piping connected to the tank that has at least 10 percent of its combined volume underground.-Tank types:...
s or through a wastestream of lead paint or lead grindings from certain industrial operations.
Lead can also be found listed as a criteria pollutant in the United States Clean Air Act section 108. Lead that is emitted into the atmosphere can be inhaled, or it can be ingested after it settles out of the air. It is rapidly absorbed into the bloodstream and is believed to have adverse effects on the central nervous system, the cardiovascular system, kidneys, and the immune system.
Biochemistry of lead poisoning
In the human body, lead inhibits porphobilinogen synthasePorphobilinogen synthase
Porphobilinogen synthase synthesizes porphobilinogen through the asymmetric condensation of two molecules of aminolevulinic acid...
and ferrochelatase
Ferrochelatase
Ferrochelatase is an enzyme that catalyses the terminal step in the biosynthesis of heme, converting protoporphyrin IX into heme. It catalyses the reaction:A ferrochelatase enzyme consists of 497 amino acid residues with a m.w...
, preventing both porphobilinogen
Porphobilinogen
Porphobilinogen is a pyrrole involved in porphyrin metabolism.It is generated by aminolevulinate and the enzyme ALA dehydratase. PBG is then converted into hydroxymethyl bilane by the enzyme porphobilinogen deaminase, also known as hydroxymethylbilane synthase.Acute intermittent porphyria causes...
formation and the incorporation of iron
Iron
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metal in the first transition series. It is the most common element forming the planet Earth as a whole, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust...
into protoporphyrin IX
Protoporphyrin IX
Protoporphyrin IX, in the metabolism of porphyrin, is created by the enzyme protoporphyrinogen oxidase.-Heme b biosynthesis:In heme biosynthesis, the enzyme ferrochelatase converts it into heme b Protoporphyrin IX, in the metabolism of porphyrin, is created by the enzyme protoporphyrinogen...
, the final step in heme
Heme
A heme or haem is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Not all porphyrins contain iron, but a substantial fraction of porphyrin-containing metalloproteins have heme as their prosthetic group; these are...
synthesis. This causes ineffective heme synthesis and subsequent microcytic anemia
Microcytic anemia
Microcytic anemia is a generic term for any type of anemia characterized by small red blood cells. The normal mean corpuscular volume is 76-100 fL, with smaller cells as macrocytic....
. At lower levels, it acts as a calcium analog, interfering with ion channels during nerve conduction. This is one of the mechanisms by which it interferes with cognition. Acute lead poisoning is treated using disodium calcium edetate: the calcium chelate of the disodium salt of ethylene-diamine-tetracetic acid (EDTA
EDTA
Ethylenediaminetetraacetic acid, widely abbreviated as EDTA , is a polyamino carboxylic acid and a colourless, water-soluble solid. Its conjugate base is named ethylenediaminetetraacetate. It is widely used to dissolve limescale. Its usefulness arises because of its role as a hexadentate ligand...
). This chelating agent has a greater affinity for lead than for calcium and so the lead chelate is formed by exchange. This is then excreted in the urine leaving behind harmless calcium.
Leaching of lead from metal surfaces
The Pourbaix diagramPourbaix diagram
In chemistry, a Pourbaix diagram, also known as a potential/pH diagram, maps out possible stable phases of an aqueous electrochemical system. Predominant ion boundaries are represented by lines. As such a Pourbaix diagram can be read much like a standard phase diagram with a different set of axes...
below shows that lead is more likely to corrode in a citrate medium than it is in a non-complexing medium. The central part of the diagram shows that lead metal oxidizes more easily in the citrate medium than in normal water.
 |
 |
| The Pourbaix diagram for lead in a non-complexing aqueous medium (e.g., perchloric acid Perchloric acid Perchloric acid is the inorganic compound with the formula HClO4. Usually encountered as an aqueous solution, this colourless compound is a strong acid comparable in strength to sulfuric and nitric acids. It is a powerful oxidizer, but its aqueous solutions up to appr. 70% are remarkably inert,... /sodium hydroxide) |
The Pourbaix diagram Pourbaix diagram In chemistry, a Pourbaix diagram, also known as a potential/pH diagram, maps out possible stable phases of an aqueous electrochemical system. Predominant ion boundaries are represented by lines. As such a Pourbaix diagram can be read much like a standard phase diagram with a different set of axes... for lead in citric acid/citrate |
In a Pourbaix diagram, the acidity is plotted on the x axis using the pH scale, while how oxidizing/reducing nature of the system is plotted on the y axis in terms of volts relative to the standard hydrogen electrode
Standard hydrogen electrode
The standard hydrogen electrode , is a redox electrode which forms the basis of the thermodynamic scale of oxidation-reduction potentials...
. The diagram shows the form of the element which is most chemically stable at each point, it only comments on thermodynamics
Thermodynamics
Thermodynamics is a physical science that studies the effects on material bodies, and on radiation in regions of space, of transfer of heat and of work done on or by the bodies or radiation...
and it says nothing about the rate of change (kinetics
Chemical kinetics
Chemical kinetics, also known as reaction kinetics, is the study of rates of chemical processes. Chemical kinetics includes investigations of how different experimental conditions can influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition...
).
Exposure pathways
Exposure to lead and lead chemicals can occur through inhalation, ingestion and dermal contact. Most exposure occurs through ingestion or inhalation; in the U.S. the skin exposure is unlikely as leaded gasoline additives are no longer used. Lead exposure is a global issue as lead mining and lead smelting are common in many countries. Most countries have stopped using lead-containing gasoline by 2007.Lead exposure mostly occurs through ingestion. Lead paint is the major source of lead exposure for children. As lead paint deteriorates, it peels, is pulverized into dust and then enters the body through hand-to-mouth contact or through contaminated food, water or alcohol. Ingesting certain home remedy medicines may also expose people to lead or lead compounds. Lead can be ingested through fruits and vegetables contaminated by high levels of lead in the soils they were grown in. Soil is contaminated through particulate accumulation from lead in pipes, lead paint and residual emissions from leaded gasoline that was used before the Environment Protection Agency issue the regulation around 1980.
Inhalation is the second major pathway of exposure, especially for workers in lead-related occupations. Almost all inhaled lead is absorbed into the body, the rate is 20–70% for ingested lead; children absorb more than adults.
Dermal exposure may be significant for a narrow category of people working with organic lead compounds, but is of little concern for general population. The rate of skin absorption is also low for inorganic lead.
According to Agency for Toxic Substance and Disease Registry, a small amount of lead (1%) will store itself in bones and the rest will be excreted through urine and feces within a few weeks of exposure. Children have a harder time excreting lead. Only about 32% of lead will be excreted by a child.
Occupational exposure
Lead is widely used in the production of batteries, metal products (solder and pipes), ammunition and devices to shield X-rays leading to its exposure to the people working in these industries. Use of lead in gasolineGasoline
Gasoline , or petrol , is a toxic, translucent, petroleum-derived liquid that is primarily used as a fuel in internal combustion engines. It consists mostly of organic compounds obtained by the fractional distillation of petroleum, enhanced with a variety of additives. Some gasolines also contain...
, paints and ceramic products, caulking
Caulking
Caulking is one of several different processes to seal joints or seams in various structures and certain types of piping. The oldest form of caulking is used to make the seams in wooden boats or ships watertight, by driving fibrous materials into the wedge-shaped seams between planks...
, and pipe solder has been dramatically reduced in recent years because of health concerns. Ingestion of contaminated food and drinking water is the most common source of lead exposure in humans. Exposure can also occur via inadvertent ingestion of contaminated soil/dust or lead-based paint.
Testing
Water contamination can be tested with commercially available kits. Analysis of lead in whole blood is the most common and accurate method of assessing lead exposure. Erythrocyte protoporphyrin (EP) tests can also be used to measure lead exposure, but are not as sensitive at low blood lead levels (<0.2 mg/L). Lead in blood reflects recent exposure. Bone lead measurements are an indicator of cumulative exposure. While measurements of urinary lead levels and hair have been used to assess lead exposure, they are not reliable.Pharmaceuticals also require laboratory testing for heavy metals such as lead. The component limit of lead (1.0 μg/g) is a test benchmark for pharmaceuticals, representing the maximum daily intake an individual should have. However, even at this low level, a prolonged intake can be hazardous to human beings.
See also
- Adult Blood Lead Epidemiology and SurveillanceAdult Blood Lead Epidemiology and SurveillanceThe US National Institute for Occupational Safety and Health funds the Adult Blood Lead Epidemiology and Surveillance program, a state-based surveillance program of laboratory-reported adult blood lead levels...
- Lead-Free Toys Act
- Medical geologyMedical geologyMedical geology is an emerging interdisciplinary scientific field consisting of those aspects of geology as they affect human, animal and plant health.Examples include:*Lead and other heavy metal exposure resulting from dust and other particulates...
- PlumbosolvencyPlumbosolvencyPlumbosolvency is the ability of a solvent, notably water, to dissolve lead. In the public supply of water this is an undesirable property. In consumers' premises plumbosolvent water can attack lead pipes and any lead in solder used to join copper pipes, leading to increased lead levels at the...
- RoHS directiveRestriction of Hazardous Substances DirectiveThe Directive on the restriction of the use of certain hazardous substances in electrical and electronic equipment 2002/95/EC was adopted in February 2003 by the European Union. The RoHS directive took effect on 1 July 2006, and is required to be enforced and become law in each member state...
- Banning of leaded petrol
External links
- Toxicological Profile for Lead (Update) Agency for Toxic Substances and Disease Registry (ATSDR). 2007. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service.
- ATSDR Case Studies in Environmental Medicine: Lead Toxicity U.S. Department of Health and Human Services
- Chemistry in its element podcast (MP3) from the Royal Society of ChemistryRoyal Society of ChemistryThe Royal Society of Chemistry is a learned society in the United Kingdom with the goal of "advancing the chemical sciences." It was formed in 1980 from the merger of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society and the Society for Analytical Chemistry with a new...
's Chemistry WorldChemistry WorldChemistry World is a monthly chemistry news magazine published by the Royal Society of Chemistry. The magazine addresses current events in world of chemistry including research, international business news and government policy as it affects the chemical science community, plus the best product...
: Lead - Lead-Free Wheels
- National Lead Free Wheel Weight Initiative| Waste Minimization|Wastes|US EPA

