
Nitrite
Encyclopedia

Ion
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving it a net positive or negative electrical charge. The name was given by physicist Michael Faraday for the substances that allow a current to pass between electrodes in a...
has the chemical formula
Chemical formula
A chemical formula or molecular formula is a way of expressing information about the atoms that constitute a particular chemical compound....
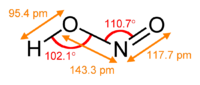
NO2−. The anion is symmetric with equal N-O bond lengths and a O-N-O bond angle of ca. 120°. On protonation the unstable weak acid nitrous acid
Nitrous acid
Nitrous acid is a weak and monobasic acid known only in solution and in the form of nitrite salts.Nitrous acid is used to make diazides from amines; this occurs by nucleophilic attack of the amine onto the nitrite, reprotonation by the surrounding solvent, and double-elimination of water...
is produced. Nitrite can be oxidised or reduced, with product somewhat dependent on the oxidizing/reducing agent. The nitrite ion is an ambidentate ligand and is known to bond to metal centres in at least five different ways. Nitrite is important in biochemistry as a source of the vasodilator nitric oxide
Nitric oxide
Nitric oxide, also known as nitrogen monoxide, is a diatomic molecule with chemical formula NO. It is a free radical and is an important intermediate in the chemical industry...
. Nitrites are used for curing meat. In organic chemistry the NO2 group is present in nitrous acid esters and nitro compounds.
Nitrite salts
Sodium nitriteSodium nitrite
Sodium nitrite is the inorganic compound with the chemical formula NaNO2. It is a white to slight yellowish crystalline powder that is very soluble in water and is hygroscopic...
is made industrially by passing nitrous fumes into aqueous sodium hydroxide or sodium carbonate
Sodium carbonate
Sodium carbonate , Na2CO3 is a sodium salt of carbonic acid. It most commonly occurs as a crystalline heptahydrate, which readily effloresces to form a white powder, the monohydrate. Sodium carbonate is domestically well-known for its everyday use as a water softener. It can be extracted from the...
solution.
- NO + NO2 + 2NaOH (or Na2CO3) → 2NaNO2 +H2O ( or CO2)
The product is purified by recrystallization. Alkali metal nitrites are thermally stable up to and beyond the melting point (441°C for KNO2). Ammonium nitrite
Ammonium nitrite
Ammonium nitrite, NH4NO2, is the ammonia salt of nitrous acid. It is used as a rodenticide, microbiocide and agricultural pesticide, and is acutely toxic to both humans and aquatic organisms.-Preparation:...
can be made from dinitrogen trioxide
Dinitrogen trioxide
Dinitrogen trioxide is the chemical compound with the formula N2O3. This deep blue liquid is one of binary nitrogen oxides. It forms upon mixing equal parts of nitric oxide and nitrogen dioxide and cooling the mixture below −21 °C :Dinitrogen trioxide is only isolable at low...
, N2O3, which is formally the anhydride of nitrous acid.
- 2NH3 + H2O +N2O3 → 2NH4NO2
This compound may decompose explosively on heating.
In organic chemistry nitrites are used in diazotization reactions.
Structure
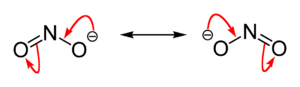
Valence bond theory
In chemistry, valence bond theory is one of two basic theories, along with molecular orbital theory, that were developed to use the methods of quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of the dissociated atoms combine to give individual chemical bonds...
it is described as a resonance hybrid with equal contributions from two canonical forms that are mirror images of each other. In molecular orbital theory
Molecular orbital theory
In chemistry, molecular orbital theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the whole molecule...
there is a sigma bond
Sigma bond
In chemistry, sigma bonds are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals. Sigma bonding is most clearly defined for diatomic molecules using the language and tools of symmetry groups. In this formal approach, a σ-bond is...
between each oxygen atom and the nitrogen atom, and a delocalized pi bond
Pi bond
In chemistry, pi bonds are covalent chemical bonds where two lobes of one involved atomic orbital overlap two lobes of the other involved atomic orbital...
made from the p orbitals on nitrogen and oxygen atoms which are perpendicular to the plane of the molecule. The negative charge of the ion is equally distributed on the two oxygen atoms. Both nitrogen and oxygen atoms carry a lone pair
Lone pair
In chemistry, a lone pair is a valence electron pair without bonding or sharing with other atoms. They are found in the outermost electron shell of an atom, so lone pairs are a subset of a molecule's valence electrons...
of electrons. Therefore the nitrite ion is a Lewis base. Moreover, it can act as an ambidentate ligand towards a metal ion, donating a pair of electrons from either nitrogen or oxygen atoms.
Acid-base properties
Nitrous acid
Nitrous acid is a weak and monobasic acid known only in solution and in the form of nitrite salts.Nitrous acid is used to make diazides from amines; this occurs by nucleophilic attack of the amine onto the nitrite, reprotonation by the surrounding solvent, and double-elimination of water...
is a weak acid
Weak acid
A weak acid is an acid that dissociates incompletely. It does not release all of its hydrogens in a solution, donating only a partial amount of its protons to the solution...
.
- HNO2 H+ + NO2-; pKaAcid dissociation constantAn acid dissociation constant, Ka, is a quantitative measure of the strength of an acid in solution. It is the equilibrium constant for a chemical reaction known as dissociation in the context of acid-base reactions...
= ca. 3.3 at 18°C
Nitrous acid is volatile; in the gas phase it exists predominantly as a trans- planar molecule. In solution it is unstable with respect to the disproportionation
Disproportionation
Disproportionation, also known as dismutation is used to describe a specific type of redox reaction in which a species is simultaneously reduced and oxidized so as to form two different products....
reaction
- 3HNO2 (aq) H3O+ + NO3- + 2NO
This reaction is slow at 0°C. Addition of acid to a solution of a nitrite in the presence of a reducing agent
Reducing agent
A reducing agent is the element or compound in a reduction-oxidation reaction that donates an electron to another species; however, since the reducer loses an electron we say it is "oxidized"...
such as iron(II) is a way to make nitric oxide
Nitric oxide
Nitric oxide, also known as nitrogen monoxide, is a diatomic molecule with chemical formula NO. It is a free radical and is an important intermediate in the chemical industry...
, NO, in the laboratory.
Oxidation and reduction
The formal oxidation stateOxidation state
In chemistry, the oxidation state is an indicator of the degree of oxidation of an atom in a chemical compound. The formal oxidation state is the hypothetical charge that an atom would have if all bonds to atoms of different elements were 100% ionic. Oxidation states are typically represented by...
of the nitrogen atom in a nitrite is +3. This means that it is can be either oxidised to oxidation states +4 and +5 or reduced to oxidation states as low as -3. Standard reduction potential
Reduction potential
Reduction potential is a measure of the tendency of a chemical species to acquire electrons and thereby be reduced. Reduction potential is measured in volts , or millivolts...
s for reactions directly involving nitrous acid are shown in the table.
| Half-reaction | E0/V |
|---|---|
| NO3- + 3H+ + 2e- HNO2 + H2O | +0.94 |
| 2HNO2+ 4H+ + 4e- H2N2O2 + 2H2O | |
| N2O4 + 2H+ + 2e- 2HNO2 | |
| 2HNO2+ 4H+ + 4e- N2O + 3H2O |
The data can be extended to include products in lower oxidation states. For example,
- H2N2O2 + 2H+ + 2e- N2 + 2H2O; E0 = 2.65V
Oxidation reactions usually result in the formation of the nitrate
Nitrate
The nitrate ion is a polyatomic ion with the molecular formula NO and a molecular mass of 62.0049 g/mol. It is the conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically-bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a...
ion, with nitrogen in oxidation state +5. For example, oxidation with permanganate
Permanganate
A permanganate is the general name for a chemical compound containing the manganate ion, . Because manganese is in the +7 oxidation state, the permanganate ion is a strong oxidizing agent. The ion has tetrahedral geometry...
can be used for quantitative analysis of nitrite, by titration.
- 5NO2- + 2MnO4- + 6H+ → 5NO3- + 2Mn2+ + 3H2O
The product of reduction reactions are various depending on the reducing agent used. With sulfur dioxide
Sulfur dioxide
Sulfur dioxide is the chemical compound with the formula . It is released by volcanoes and in various industrial processes. Since coal and petroleum often contain sulfur compounds, their combustion generates sulfur dioxide unless the sulfur compounds are removed before burning the fuel...
the products are NO and N2O; with tin(II), Sn2+, the product is hyponitrous acid
Hyponitrous acid
Hyponitrous acid is the chemical compound H2N2O2. This can be formulated as HON=NOH and is an isomer of nitramide, . It forms white crystals that are explosive when dry...
, H2N2O2; reduction all the way to ammonia occurs with hydrogen sulfide
Hydrogen sulfide
Hydrogen sulfide is the chemical compound with the formula . It is a colorless, very poisonous, flammable gas with the characteristic foul odor of expired eggs perceptible at concentrations as low as 0.00047 parts per million...
. With the hydrazinium
Hydrazine
Hydrazine is an inorganic compound with the formula N2H4. It is a colourless flammable liquid with an ammonia-like odor. Hydrazine is highly toxic and dangerously unstable unless handled in solution. Approximately 260,000 tons are manufactured annually...
cation, N2H5+, hydrogen azide, HN3, is produced
- HNO2 + N2H5+ → HN3 + H2O + H3O+
which can also further react with nitrite
- HNO2 + HN3 → N2O + N2 + H2O
This reaction is unusual in that it involves compounds with nitrogen in four different oxidation states.
Coordination complexes
The nitrite ion is known to form complexes in at least five different ways.- When donation is from nitrogen to a metal centre, the complex is known as a nitro- complex.
- When donation is from one oxygen to a metal centre, the complex is known as a nitrito- complex.
- Both oxygen atom may donate to a metal centre, forming a chelate complex.
- A nitrite ion can form an unsymmetrical bridge between two metal centres, donating through nitrogen to one metal and oxygen to the other.
- A single oxygen atom can bridge to two metal centres.
Alfred Werner
Alfred Werner
Alfred Werner was a Swiss chemist who was a student at ETH Zurich and a professor at the University of Zurich. He won the Nobel Prize in Chemistry in 1913 for proposing the octahedral configuration of transition metal complexes. Werner developed the basis for modern coordination chemistry...
studied the nitro-nitrito isomerism (1 and 2) extensively. The red isomer of cobalt pentammine with nitrite is now known to be a nitrito complex, [Co(NH3)5(ONO)]2+; it is metastable and isomerizes to the yellow, nitro complex [Co(NH3)5(NO2)]2+. An example of chelating nitrite (3) was found in [Cu(bipy)2(O2N)]NO3; bipy is the bidentate ligand 2,2'bypyridyl
Bipyridine
Bipyridines are a family of chemical compounds with the formula 2, which are formed by the coupling of two pyridine rings. Six isomers of bipyridine exist, but two isomers are prominent: 2,2'-bipyridine is a popular ligand in coordination chemistry and 4,4'-bipyridine is a precursor to the...
and the two bipy ligands occupy four coordination sites on the copper ion so the nitrite is forced to occupy two sites in order to achieve an octahedral environment around the copper ion. Examples of 4 and 5 are illustrated.
Nitrite in biochemistry
Sodium nitrite is used for the curingCuring (food preservation)
Curing refers to various food preservation and flavoring processes, especially of meat or fish, by the addition of a combination of salt, nitrates, nitrite or sugar. Many curing processes also involve smoking, the process of flavoring, or cooking...
of meat
Meat
Meat is animal flesh that is used as food. Most often, this means the skeletal muscle and associated fat and other tissues, but it may also describe other edible tissues such as organs and offal...
because it prevents bacterial growth and, in a reaction with the meat's myoglobin
Myoglobin
Myoglobin is an iron- and oxygen-binding protein found in the muscle tissue of vertebrates in general and in almost all mammals. It is related to hemoglobin, which is the iron- and oxygen-binding protein in blood, specifically in the red blood cells. The only time myoglobin is found in the...
, gives the product a desirable dark red color. Because of the toxicity of nitrite (the lethal dose of nitrite for humans is about 22 mg per kg body weight), the maximum allowed nitrite concentration in meat products is 200 ppm. Under certain conditions, especially during cooking, nitrites in meat can react with degradation products of amino acids, forming nitrosamine
Nitrosamine
Nitrosamines are chemical compounds of the chemical structure R1N-N=O, some of which are carcinogenic.-Usages:Nitrosamines are used in manufacture of some cosmetics, pesticides, and in most rubber products. -Occurrences:...
s, which are known carcinogen
Carcinogen
A carcinogen is any substance, radionuclide, or radiation that is an agent directly involved in causing cancer. This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes...
s.
Nitrite is detected and analyzed by the Griess Reaction, involving the formation of a deep red-colored azo dye upon treatment of a NO2−-containing sample with sulfanilic acid
Sulfanilic acid
Sulfanilic acid is a grey crystalline solid produced from sulfonation of aniline.It readily forms diazo compounds and is used to make dyes and sulpha drugs....
and naphthyl-1-amine in the presence of acid. Nitrite can be reduced to nitric oxide
Nitric oxide
Nitric oxide, also known as nitrogen monoxide, is a diatomic molecule with chemical formula NO. It is a free radical and is an important intermediate in the chemical industry...
or ammonia
Ammonia
Ammonia is a compound of nitrogen and hydrogen with the formula . It is a colourless gas with a characteristic pungent odour. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia, either directly or...
by many species of bacteria
Bacteria
Bacteria are a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria have a wide range of shapes, ranging from spheres to rods and spirals...
. Under hypoxic conditions, nitrite may release nitric oxide, which causes potent vasodilation
Vasodilation
Vasodilation refers to the widening of blood vessels resulting from relaxation of smooth muscle cells within the vessel walls, particularly in the large arteries, smaller arterioles and large veins. The process is essentially the opposite of vasoconstriction, or the narrowing of blood vessels. When...
. Several mechanisms for nitrite conversion to NO have been described including enzymatic reduction by xanthine oxidoreductase, nitrite reductase
Nitrite reductase
Nitrite reductase refers to any of several classes of enzymes that catalyze the reduction of nitrite. There are two classes of NIR's. A multi haem enzyme reduces NO2 to a variety of products. Copper containing enzymes carry out a single electron transfer to produce nitric oxide.- Iron based...
and NO synthase (NOS), as well as nonenzymatic acidic disproportionation
Disproportionation
Disproportionation, also known as dismutation is used to describe a specific type of redox reaction in which a species is simultaneously reduced and oxidized so as to form two different products....
.
Organic nitrites and nitro compounds
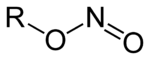
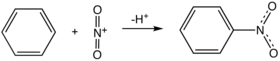
Organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, composition, reactions, and preparation of carbon-based compounds, hydrocarbons, and their derivatives...
, nitrites are ester
Ester
Esters are chemical compounds derived by reacting an oxoacid with a hydroxyl compound such as an alcohol or phenol. Esters are usually derived from an inorganic acid or organic acid in which at least one -OH group is replaced by an -O-alkyl group, and most commonly from carboxylic acids and...
s of nitrous acid and contain the nitrosooxy functional group. Nitro compounds contain the C-NO2 group. Nitrites have the general formula RONO, where R is an aryl
Aryl
In the context of organic molecules, aryl refers to any functional group or substituent derived from an aromatic ring, be it phenyl, naphthyl, thienyl, indolyl, etc....
or alkyl group. Amyl nitrite
Amyl nitrite
Amyl nitrite is the chemical compound with the formula C5H11ONO. A variety of isomers are known, but they all feature an amyl group attached to the nitrito functional group. The alkyl group is unreactive and the chemical and biological properties are mainly due to the nitrite group...
is used in medicine for the treatment of heart diseases. A classic named reaction for the synthesis of alkyl nitrites is the Meyer synthesis in which alkyl halides react with metallic nitrites to a mixture to nitroalkanes and nitrites.
Nitrobenzene
Nitrobenzene
Nitrobenzene is an organic compound with the chemical formula C6H5NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale as a precursor to aniline. Although occasionally used as a flavoring or perfume...
is a simple example of a nitro compound
Nitro compound
Nitro compounds are organic compounds that contain one or more nitro functional groups . They are often highly explosive, especially when the compound contains more than one nitro group and is impure. The nitro group is one of the most common explosophores used globally...
. In aromatic nitration reaction a C-H bond is broken leaving the two electron on the carbon atom. The two electrons are added to the nitronium ion
Nitronium ion
The nitronium ion, or sometimes the nitryl ion , , is a generally reactive cation created by the removal of an electron from the paramagnetic nitrogen dioxide molecule, or the protonation of nitric acid....
reducing it to nitrite.
External links
- Material Safety Data Sheet, sodium nitrite
- ATSDR - Case Studies in Environmental Medicine - Nitrate/Nitrite Toxicity U.S. Department of Health and Human Services (public domain)

